Table of Contents
In my opinion, the concentration of minerals by flotation is the most interesting problem in ore-dressing, and will command eventually far more consideration than it has at present. For many ores it furnishes a complete method of concentration absolutely independent of specific gravity, and thus simplifies the milling of many difficult combinations. Not having to recognize the influence of specific gravity, we are also able to dispense with some of the complications resulting from classifying-conditions.
Where flotation cannot be used for a complete separation of the minerals from the gangue, it will be found, in nearly all cases, to save those portions which are generally lost by reason of their fine state of division. For this reason, it should be looked upon mainly as an aid to general concentration, although there are many ores so well adapted to flotation that more than 90 per cent, of the valuable metals can be recovered from them in one operation by this process.
In presenting the results of my investigations in flotation, I have endeavored to theorize as little as possible. That side of the subject has been extensively discussed by others.
My earliest work with flotation was done in 1895, when I used various oils, grease, soap, etc., with results that were remarkable. I did not realize then that with water alone equally good separations could be made. Up to 1906, my endeavors were confined to attempts to keep the concentrates on the tables well wetted and away from exposure to the air. In common with others, I observed that when there was any obstruction in the wash-water on the table of sufficient size to divert the water to either side, the concentrates collected, and, on exposure to the air, would promptly float away. This convinced me that the sulphides were all good “ swimmers,” and that if, instead of endeavoring to drown them, they chance, they would all float.
 Having successfully demonstrated this fact, as well as another equally important, namely, that practically all the oxides are quickly wetted, become saturated like a sponge, and, sinking under the surface-film, become subject to gravity-conditions, I commenced my experiments.
Having successfully demonstrated this fact, as well as another equally important, namely, that practically all the oxides are quickly wetted, become saturated like a sponge, and, sinking under the surface-film, become subject to gravity-conditions, I commenced my experiments.
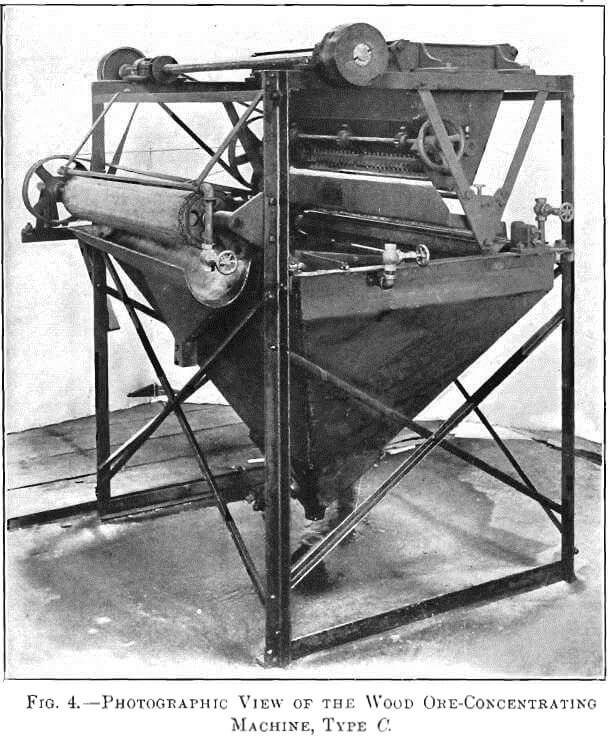
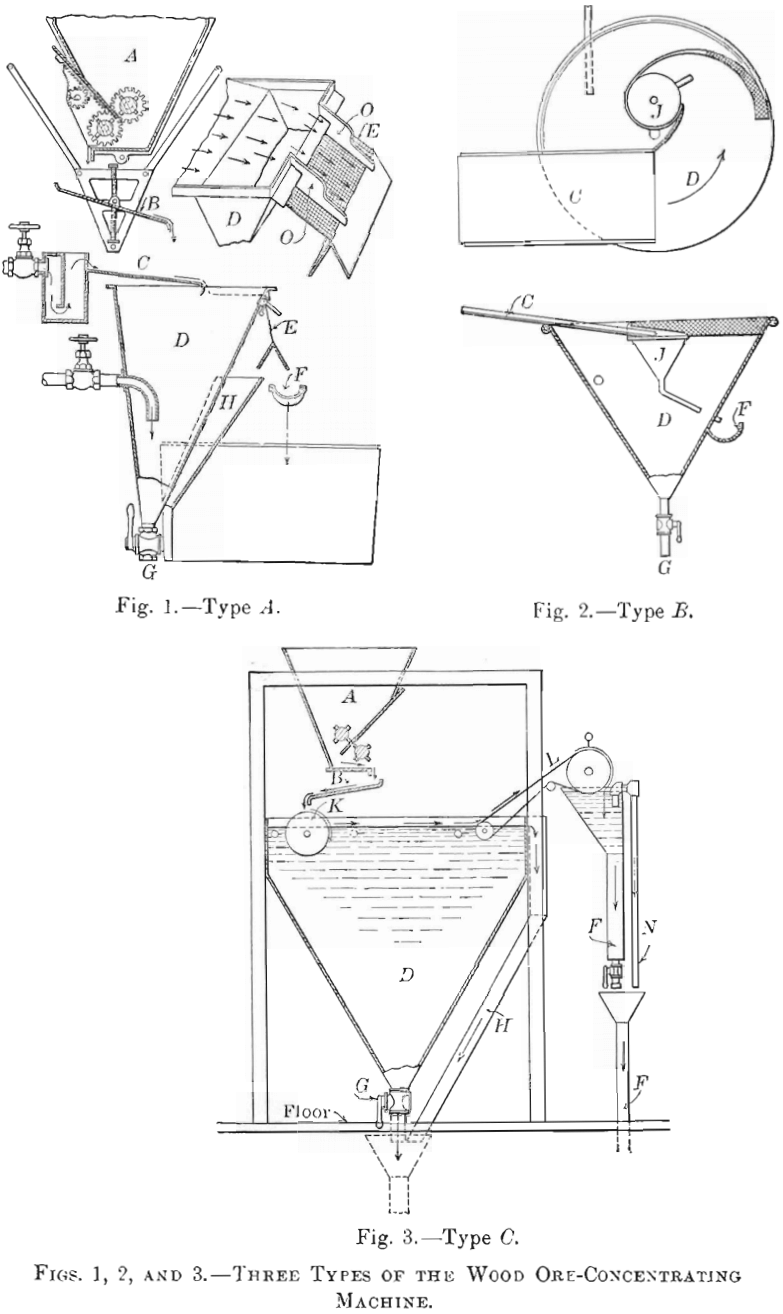
I found that if dry-crushed ore, not necessarily very fine, was gently deposited upon a swiftly-moving sheet of water, separation of the sulphides from the gangue took place. Retarding the current permitted the gangue to sink, while a film of sulphides remained on the surface. To collect this film I have devised three types of machine, shown in Figs. 1, 2, and 3. Fig. 4 is a photographic view of the type C machine.
All three types are similar so far as the system of feed is concerned. In type A, a sectional view of which is presented in Fig. 1, the unsized ore, at whatever mesh has been decided upon, and ranging from 10-to 40-mesh or finer, is delivered to a feed-hopper, A, the outlet from which is under close control, so that a uniform amount of ore can be steadily dropped upon a plate, B, vibrating 500 times per minute. From this plate the ore falls direct to the sheet of water, which flows over a plane, C, inclined at 7°. The film of sulphides crosses the tank, D, and in falling over the outlet is dewatered and split off by means of a nearly vertical screen, E. O, O, are guides or stretchers, and perform quite an important function when this type of machine is used. The elastic properties of the film are such that if these guides were not used, the sheet of water emerging from the tank would quickly assume a spiral shape, which would result in a wetting of the sulphides. These would then pass through the screen instead of over it.
The guides are so adjusted that the water intersects the screen in a line parallel to the surface of the tank. By slight adjustments one is then able to split off cleanly the film of concentrates from the water and the gangue in suspension. A separate patent was allowed for this feature. The water passing through the screen, containing more or less fine gangue in suspension, is caught at H and conducted to a launder, F, which also collects such coarse mineral and gangue as steadily issues from the bottom outlet of the tank, G, from which it passes to tables. I sometimes find it advantageous either to classify hydraulically or screen-size the tank contents en route to the table; but it is not essential in all cases, because, having removed the fine sulphides, as well as a large proportion of the larger sizes, by flotation, only the coarser mineral and the coarser gangue have to be considered, as the fine gangue in suspension passes off with the wash-water.
The feed of the type B machine, Fig. 2, is delivered over the inclined plane C to a conical tank, D, in which a rapidly-revolving motion with vortical effect is given to the surface of the water by means of a water-jet against the side of the tank. The feed falls upon an inclined sheet of water, The film of sulphides is guided by means of partly-submerged vertical screens, E, to a funnel, J, the outlet of which penetrates the side of the tank, where the concentrates are delivered to a launder, F.
The output of a tank 5 ft. in diameter will exceed 0.5 ton per hour on a 20-mesh ore that is adapted to flotation. Necessarily, a small amount of water is required to carry the film into the funnel. The film, having a definite thickness, is probably carried on an amount of water of two or more times the volume of the concentrate. As this water carries more or less gangue in suspension, it makes the concentrates siliceous. In certain sections of Mexico, where copper sulphides are in demand and silica is not objectionable, the conical-tank plan meets the requirements of the situation; but this form would not be satisfactory, for instance, in Utah, where a high-grade copper-concentrate is of more importance than the highest possible recovery. In Utah there is a penalty imposed by the smelters for silica above a certain amount, a condition which does not exist in Mexico.
In the type C machine, Fig. 3, the 20- to 40-mesh ore is dropped from the vibrating plate, B, to a corrugated rubber- or canvas-covered cylinder, K, which is revolving in water. Enough water is elevated above the water-level of the tank to wet the gangue and float the sulphides. This sulphide film passes from the cylinder over the tank-surface to an endless canvas belt, A, which emerges from the tank and is controlled by sprockets. Water-jets gently force the concentrates upon the canvas, but, as the canvas is elevated, the water drains back to the tank, carrying with it any gangue in suspension. The concentrates are deposited in another tank and collected in a launder, F. An automatic siphon, N, prevents an overflow of concentrates from the tank after they are discharged from the belt. G is the outlet-valve controlling the discharge of tailings after flotation. In most cases the tailings pass to a Wilfley table. Quite frequently, however, the recovery made by flotation makes any additional treatment unnecessary.
As clean wash-water is used at the delivery end of the take-off belt to remove the concentrates, they have a minimum silica- content. The difference between type B and type C in this respect was shown by comparative tests. The crude ore used in each test assayed 2.63 per cent, of copper. The B machine saved 88 per cent, and gave concentrates assaying 18 per cent, of copper, with 28 per cent, of SiO2, while the G machine saved 93 per cent., giving concentrates assaying 27 per cent, of copper, with 3.6 per cent, of SiO2, both tests being made by flotation alone.
As the corrugations of the rubber-covered cylinder are only adapted to comparatively fine ore, say 20-mesh and finer, they would not bring up enough water to saturate particles that were larger than the corrugations; hence, in treating 8- or 10- mesh ore, all of such particles would not reach the water, and other dry ore would fall on top of them, causing an unclean concentrate. To prevent such conditions in treating coarse ores, the cylinder is removed, and a rapid impetus is given to the surface of the water by means of water-jets from a pipe just in advance of the position formerly occupied by the cylinder.
An ore suitable for jigging, in which the sulphides are liberated at 4-mesh, can be fed, either sized or unsized, direct to the tank. The treatment of coarse ore, say from 4- to 12-mesh, is performed for the purpose of floating off fine particles of sulphides which mechanically adhere to the coarser sizes. I have carefully dry-screened ore from 4- to 20-mesh and then subjected these coarse sizes to flotation, the result being a high-grade product that would add 5 per cent, to the general recovery.
During my experimental work on more than 800 separate samples, many interesting incidents were observed, among which I note the following:
If, after grinding a given ore to the right size, it is at once floated, from 30 to 85 per cent, by weight of its sulphide particles are likely to float. If another portion, prepared at the same time and under the same conditions, is set aside for several days and then treated, less than one-third of what floated before will remain on the surface. This applies particularly to the copper sulphides, and must be due to partial oxidation, although such a change is not visible to the eye. This behavior of copper-ores is more pronounced if steam-heat be applied in drying a previously-made concentrate for flotation. It is therefore not advisable to regrind wet jig-tailings and dry them for flotation. To secure the best possible flotation from copper-ores, they must be ground directly to the required mesh for separation of the mineral from the gangue, and promptly milled.
Curiously enough, this state of affairs does not exist in the treatment of some other complex sulphides, for instance, those containing galena and the non-magnetic resin blend. At 40- mesh a 10-per cent, lead-ore, with 20 per cent, of zinc, will produce a flotation-concentrate accounting for 80 per cent, of the lead, assaying from 56 to 60 per cent, of Pb and 7 per cent, of Zn. If the tailings, after flotation, are passed over a Wilfley table, a high-grade coarse lead-concentrate is easily cut out from the zinc and silica. The removal of the silica from the zinc-middlings enables us to obtain a high-grade zinc-concentrate. Now, if this blende, which sank in the tank during flotation, be dried and re-treated by flotation, nearly all of it will float, while the accompanying silica will sink, leaving a high-grade product. This particular effect is not so pronounced when the blende is of the magnetic or so called black-jack variety.
I have noted in the treatment of copper-ores that the tendency to float decreases as the sulphur-content diminishes. More than 90 per cent, of the copper of chalcopyrite ore with a hard white quartz gangue can be saved with less than 5 per cent, of silica in the concentrate by flotation alone; but if bornite or chalcocite is present, the proportions of these minerals saved are much smaller than of chalcopyrite. If chalcocite contains a small percentage of chalcopyrite, practically all of the latter will float and some of the fine chalcocite with it. This leaves the coarse chalcocite in good shape for final table-separation.
Most of the magnetic minerals will sink, so that a good flotation-concentrate can sometimes be made from a mixture of chalcopyrite associated with a magnetic blende. Magnetite will sink and leave suspended on the surface any fine sulphides that may be with it, as well as the metals, such as gold, silver, platinum, etc. To obtain the best effects for the fine metals, we sometimes modify the film by using a minute amount of oil. The oil being used purely as a conveyor, and not as an enveloping element, my patents do not conflict with the oil-systems. My main idea is the use of water alone. I rarely use oil and never acid or gas.
Judging from the appearance of a 20-mesh flotation-concentrate, the inexperienced observer assumes, from the smooth surface of the film, that only the extremely fine sulphides are being recovered. A screen-analysis of the concentrate, however, shows that more than 10 per cent, of the concentrate is between 20- and 40-mesh. Apparently the elasticity of the film is such that it supports the coarser particles, but not rigidly, so that only the apex of each piece of sulphide is shown, and as it lies along with the fines, a uniform, smooth surface is presented.
The strength of the film is illustrated in another way. If particles of dry ore are placed upon the film of sulphides, they will be thrown off by the rebound of the film as it passes over the curve of the dam, the effect being practically the same as the repulsion of highly-conductive minerals in passing over an electrostatically-charged roller.
The elastic properties of the film are plainly shown during the operation. The sulphide film spreads out rapidly over the tank-surface. If its passage is restricted, it will immediately be compressed into ridges, which in time become sufficiently heavy to sink of their own weight. Also, before sinking, the film will creep up vertically on the corrugated roller.
Violent agitation of the film must be avoided, or the particles will become wet and sink. Nevertheless, the tenacity of the film is remarkable.
As long as the feed is continuous, the sulphides on the film easily slip over to the take-off belt. This action is assisted by a gentle water-pressure directly in front of the belt. A gentle current of air also produces the same result.
I have alluded to oxidizing influences preventing or interfering with flotation-results in the treatment of copper sulphides. With several other sulphides this is not the case, so that flotation can frequently be applied, after amalgamation and concentration, as a more economical method of recovery than cyanidation of the tailings.
For instance, I had a $20 gold-ore, of which $15, or 75 per cent., was removed by amalgamation and concentration. Of the $5 left in the tailings, 93 per cent, was extracted by cyanide, but at a cost of $2.75, by reason of the high cyanide-consumption, caused by values still locked in the fine sulphides. The same tailing’s, assaying $5, were dried and flotated, producing a 10-oz. gold-concentrate and leaving 80 cents per ton in the final tailings. The cost was far less than that of cyanide treatment.
Nearly all the average free-gold ores of California contain arsenical pyrites, and this, in turn, carries gold that is not free. Such ores, if crushed dry and treated by flotation, yield high- grade concentrates, accounting for from 10 to 20 per cent, of the total valuable metals in the ore. After flotation, the free gold can be amalgamated as usual. I have found that all that is necessary is to feed over an amalgamated plate, which has an inclination of 7° and is covered by water.
Such flotation-concentrates of arsenical pyrites frequently assay many thousands of dollars per ton, and constitute an important item in the total recovery. A considerable percentage of the values in some varieties of wet-crushed ores, particularly of copper and arsenical sulphides in a hard gangue, will float. While we cannot do anything with sulphides in suspension, we can readily save all floating slimes from wet-crushing by this same general system, and with a simple device placed in any launder below the mill, provided the surface-conditions are not rough.
All over the world, valuable ores, such as sulphides of silver, gray copper, galena, etc., are frequently found in a matrix of baryta, or associated with heavy garnet. The specific gravity of such gangues practically prohibits either jigging or table- separation ; but when this method of ore-dressing is applied to them, from 80 to 90 per cent, of their values can be recovered at one simple operation. A recent example of this was a 1,200-oz. silver-concentrate made from a Mexican ore containing 78 per cent, of BaSO4. Another, from Canada—a crystallized barite containing 10 per cent, of Pb—gave a 90-per cent, recovery in a 70-per cent, lead-product. Many mines containing ores of such composition have been abandoned because of the closeness of the specific gravities.
We have had several ores of nearly clean pyrrhotite containing a small percentage of chalcopyrite. Most of the latter will float by itself. If the remainder is concentrated, the ratio of concentration is too low to make a valuable copper-product; but if this concentrate is dried and passed through a magnetic separator, such as the Wetherill or Dings, at a low amperage, the pyrrhotite is lifted off and the non-magnetic tailings will be a clean chalcopyrite. This, combined with the flotation-concentrate, gives a total high extraction.
The capacity of the series of flotation-machines here shown is variable, depending upon several conditions, among which are the nature of the gangue, the size of the ore, and the concentration-ratio. A standard machine treating a 20-mesh quartz ore, using a 3-ft. width of feed and having a 4-ft. take-off belt, will vary in capacity from 1.000 to 2,000 lb. per hour, unless the ratio of concentration is low, in which case the capacity will be smaller. Some ores that possess an easily wetted gangue and call for a high concentration-ratio can be fed rapidly at 20-, 30-, or 40-mesh.
For instance, a 1 or 2 per cent, molybdenite ore in a quartz gangue will give a clean concentrate, even if the ore is fed several times faster than an ordinary sulphide ore; and the same is true of graphite. In a special form of the machine, designed for the concentration of molybdenite and graphite, one or two partly-submerged cylinders, covered with either canvas or fine wire screen, are inserted in the tank. These cylinders revolve in the same direction and at the same rate of speed as the main take-off belt or belts. The film-concentrate is lifted from the tank, and passes over the rollers down to the tank-level again. With each elevation partly-saturated gangue becomes thoroughly wetted, and drops off into the tank without any apparent disturbance or wetting of the concentrate. Such action naturally raises the grade of the concentrate. In leaving the take-off belt, it is not washed off by direct water-jets, as is done with other ores, but the take-off belt is allowed to discharge its load at water-level in a shallow compartment, and the concentrate gently floats off to a collecting-launder.
In the flotation-treatment of molybdenite it frequently happens that copper sulphides, iron pyrite, and other impurities, are present, and must be removed to make a salable product. If copper and iron are present, they are re-concentrated on a Wilfley table, so handled that the tailings will contain the molybdenite. Another way is to dry the flotation-concentrate, give it a gentle roast, and pass it through a magnetic separator, to remove the copper and iron. Molybdenite- and graphite- concentrates are frequently contaminated by mica. This in some cases can also be taken out by the magnetic separator, or by re-flotation with the water at 110° F. It can also be removed electrostatically.
Objections are made to the drying of flotation-concentrates on accouut of their supposed fineness, but this is a mistake, as mentioned previously. There should be no more difficulty in drying concentrates of this nature than those produced by vanners or ordinary slime-tables. By alternating settling- tanks, which can be siphoned, or by using filter-presses, they can be constantly dewatered. They can be briquetted if necessary.
There are, in Mexico and elsewhere, dry-crushing mills that use magnetic separators to remove large amounts of gangue in the shape of garnets, siderite, etc., in order to get more favorable conditions for gravity-separations on the tables for the lead and zinc. If a flotation-machine be placed so that it will take the non-magnetic tailings before they reach the table, a high-grade lead-product results, and aids in the general separation of the coarse lead and zinc, improving each from 15 to 20 per cent.
Flotation will be found to be of the greatest benefit when applied to ores that have the largest losses in milling, as well as to certain ores that are virtually not subject to wet concentration on account of small differences in specific gravity.
The proper preparation of ores for flotation is essential. For instance, if a small sample is pulverized unnecessarily flue, in such a state as would result from the use of an ordinary muller, poor returns may be expected, but if the same ore is shattered by pounding on an iron plate, most favorable products will ensue, as the crystals retain more of their original shape.
For this reason, I have found that ores should be prepared in ball-mills, or by rolls, rather than in any machine that grinds much of the gangue to an impalpable dust, in which condition both the gangue and the mineral of many ores will float. Flotation-treatment of definitely-sized ore will materially increase the capacity of the large sizes by themselves, but mechanical difficulties are encountered in feeding the smaller sizes by themselves, and the capacity is also much reduced; hence it is advantageous, after having decided upon the proper mesh to liberate the sulphides from the gangue, to feed them all at once without previous sizing.
I wish, also, to place special emphasis upon the property of easy saturation or wetting of nearly all of the oxides. The only one that has surprised us and floated in appreciable proportions is the specular hematite in certain New Mexico zinc-ores, which floats up to 10 or 20 per cent, by weight. This is largely due to the tabular shape of its crystals. After magnetic removal of the iron, the non-magnetic tailings assay from 45 to 50 per cent, of zinc.
Simple as these flotation-devices are, it has taken several years to secure patents, both domestic and foreign, as well as to develop the general standard type of machine now on the market. It weighs 1,000 lb. net; measures 4 by 5 by 6 ft. Ready for mule-back transportation, its weight is 1,100 lb. It requires 0.25 h-p. and less water than is used in wet concentration.
Sulphides of the following metals are particularly favorable for flotation methods: Copper, lead, silver, antimony, bismuth, mercury, arsenic, molybdenum, zinc, iron, nickel, etc., as well as tellurides, graphite, gold, and platinum.
When tellurides are present, a high-grade flotation-concentrate is obtained and the tailings are left in excellent shape for cyanidation. I have proved this commercially on ores from Boulder county, Colo.
Molybdenite Flotation
The concentration of molybdenite by flotation is decidedly interesting, in that it furnishes illustrations of several of the peculiarities of surface-tension. For instance, in the recent flotation of a Canadian ore of this nature, with a quartz-schist gangue, which assayed 1.87 per cent, of MoS2, I obtained an apparently pure film of MoS2, which, when assayed, was found to contain only 45 31 per cent, of MoS2, and 41.97 per cent, of insoluble residue. On examination, the under side of the film was seen to be composed of fine mica scales, which must have adhered to the flakes of MoS2. My first conclusion, that the mica was simply enveloped in a coating of MoS2, was not correct. The recovery in this particular case was 79.08 per cent. The test was made with water having a temperature of 56° F.
The above examination was followed by that of an 8.65-per cent, molybdenite ore from Alaska. This was a complex ore, containing pyrrhotite and magnetite, and low in silica. The flotation-concentrate, at 40-mesh, amounted to 23.4 per cent, by weight. As it assayed only 30 per cent, of MoS2, a recovery of 79 per cent., it was evidently contaminated by other minerals that had floated.
Another sample of the same ore, lightly roasted, gave a flotation-concentrate assaying 81.45 per cent, of MoS2, the ratio of concentration being 35.71 : 1, but the saving was only 26.3 per cent. This indicates that either some of the molybdenum is present in the original ore as an oxide, or that the roasting was carried too far and oxidized it. In the first test the saving was 79 per cent., assaying 30 per cent, of MoS2, while the second trial recovered but 26 per cent., assaying 81.45 per cent. It is possible, therefore, that by obtaining the right roasting-conditions, such an ore could be profitably treated.
The concentrates from one test of this ore assayed 1.86 per cent, of copper. After a gentle roast, this was removed by the Wetherill separator, leaving only a trace of copper, and with no material loss of MoS2.
A certain Alaskan ore recently treated by me in commercial quantities furnishes an illustration of the extreme sensitiveness of chalcopyrite to oxidizing influences. It contained 4 per cent, of copper, closely associated with a heavy pyrrhotite in a siliceous gangue. Careful work on this ore by jigging, tabling, regrinding of jig-tailings, all followed by canvas slime-table treatment, gave a 15 per cent, copper-concentrate, with a recovery of 62 per cent. Direct table-concentration made a 16 per cent, copper-concentrate, saving 47 per cent. The same ore, by flotation alone, made a 25-per cent, copper-concentrate, accounting for 69 per cent, of the copper. To this was added 11 per cent, from the table-work, a total of 80 per cent. In making this examination the ore was dry-crushed at 8-mesh, and the from 8- to 20-mesh product (dry-screened) was passed through the flotation-machine, resulting in a high-grade concentrate, which added 4.25 per cent, to the total recovery. This proportion came from the mechanically adhering fine sulphides, and would be lost in ordinary jig-work. The from 8- to 20- mesh jig-tailings of this test were dried and reground for flotation ; but on account of the oxidation not a particle floated.
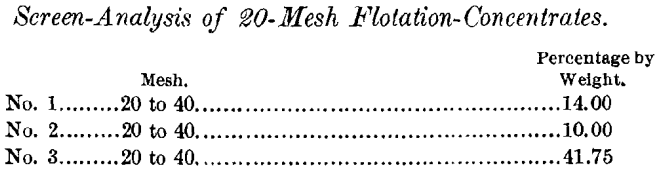
Many similar examples could be cited showing that flotation largely reduces the proportions going to the tables, thereby increasing the efficiency of the latter.
Table I. gives the results obtained by flotation alone. All of the ores tried were difficult ones to treat, and several of them were not susceptible to gravity-separation. The recovery by flotation alone, in many instances, exceeded the returns from the best possible treatment in the wet way.
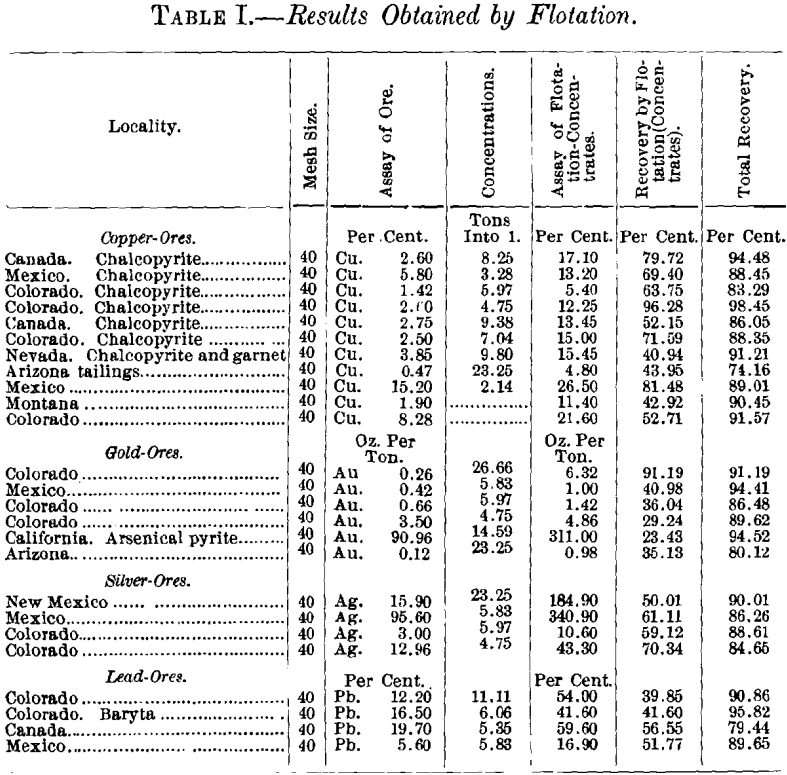
Copper Ores
Alaska.—30 mesh, 4.08 per cent, of copper. Close classification, jigging, table-concentration, with regrinding of jig-tailings, gave 65 per cent, of the copper, assaying 15.66 per cent, of Cu. Ratio, 5.9 tons to 1.
By flotation at 30-mesh—the same ore—the concentrate as-sayed 25.03 per cent, of Cu, recovering 73 per cent, at a ratio of 8.4 into 1. The Wilfley concentrate added to this 7.31 per cent, more, assaying 6.32 per cent, of Cu, ratio 21.19 to 1, a total recovery of 80.31 per cent. When treated at 40-mesh by flotation alone, the recovery exceeds the above total.
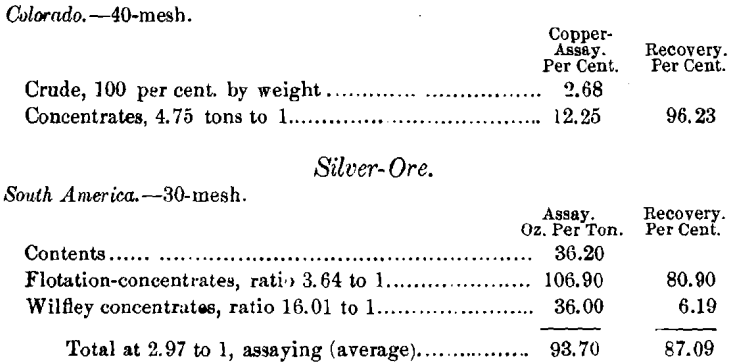
A second test of this ore gave 91 per cent. of its gold-content, 96.3 per cent. of the silver, 93.9 per cent. of the lead, and 84.41 per cent. of the copper. Several other combination methods applied to this ore failed to produce satisfactory commercial products.
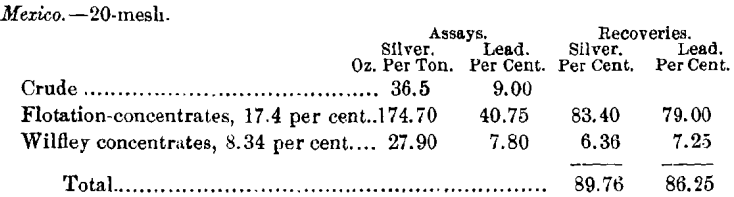
The ore from Mexico when treated most carefully by wet- concentration gave 75.22 per cent, of the silver and 74.38 per cent, of the lead, both silver and lead being of much lower
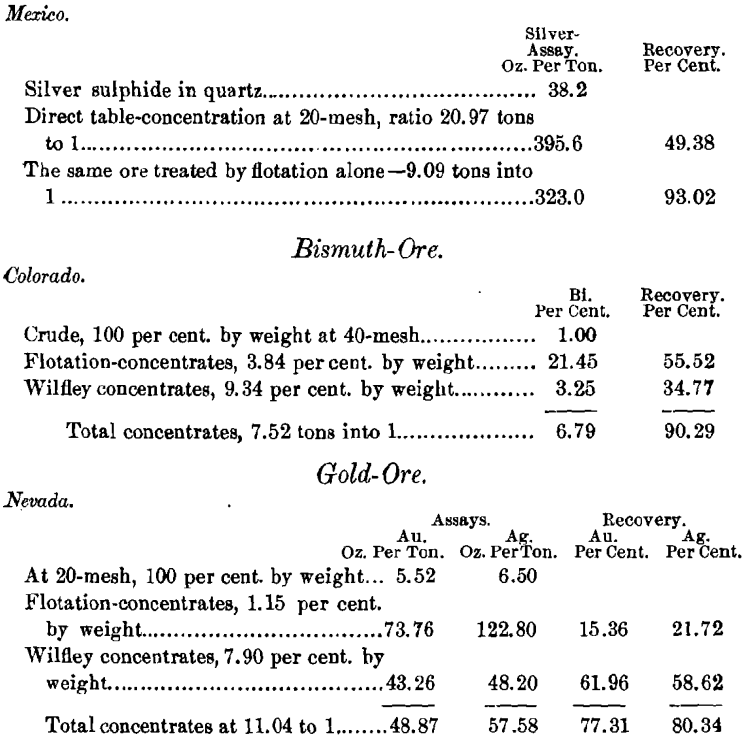
Left in the tailings—$25 in gold and 69 cents in silver, in excellent condition for cyanide treatment, the sulphides having been removed.
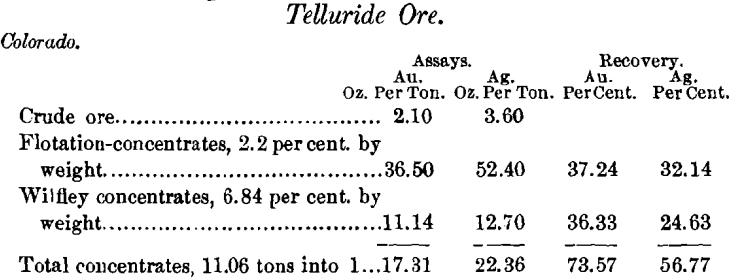
With extraction of the tellurides the values remaining in the tailings were successfully cyanided.

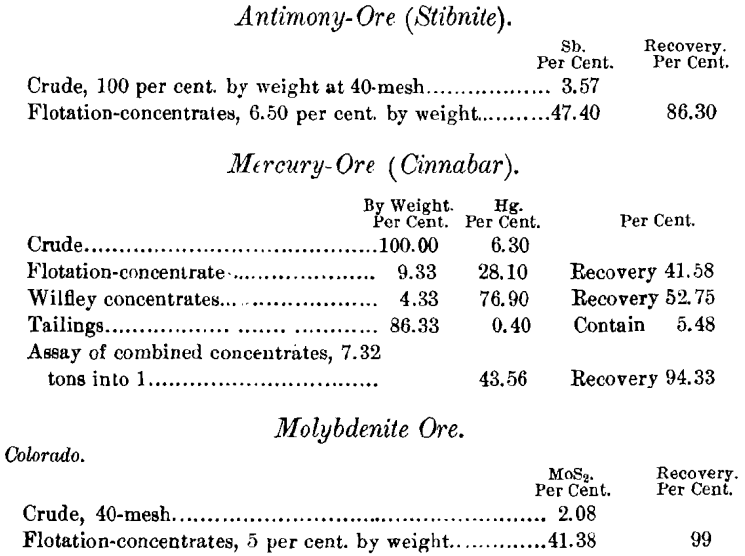
Re-treatment would materially increase the grade with little loss of MoS2.