Table of Contents
The Bureau of Mines Tuscaloosa Metallurgy Research Laboratory is conducting waste utilization research concerned with the development of building products from metal-free inorganic materials separated from municipal incinerator residues in the Bureau’s continuous residue-processing plant at the Edmonston Laboratory of the College Park Metallurgy Research Center.
This is the third in a series of publications reporting results of research with waste glass carried on at the Tuscaloosa Laboratory. The first phase of the investigation was directed to the production of building brick, an industry that consumes more than 17 million tons of siliceous raw materials in producing nearly 7 billion bricks per year. The second phase of the investigation was directed to the production of glass wool thermal insulation. The present report, which represents the third phase of the investigations, was undertaken to demonstrate how the fluxing properties of waste glass might be utilized to decrease the required firing temperature and/or firing time of structural clay products made, from common clays, thereby lowering manufacturing costs, recycling waste glass, and conserving fuel.
About 15 million tons of glass is discarded in municipal wastes annually; of this, about 2 million tons pass through incinerators and can be recovered by ore processing techniques developed at the Bureau of Mines Edmonston Laboratory. Operating at a feed rate of 1,000 pounds per hour, the continuous-processing plant has recovered 578 pounds of cullet-grade glass and 414 pounds of waste glass per ton of residue. The waste material of immediate interest was that fraction composed principally of glass so contaminated as to be worthless for cullet.
Raw Materials
Waste Glass
The glass used in fluxing tests is one of the waste products that remains after free metal and cullet-grade glass have been removed from incinerator residues in the Bureau of Mines continuous-processing plant at Edmonston, Md. The minus 20-mesh product is a mixture of multicolor glass, slag, ceramics, and stone. In mixtures of glass bonded with clay, fine glass acts as a flux-that lowers the maturing temperature of the body. The slag darkens red-firing clays and tends to give tan-firing clays a gray cast. Generally, ceramic materials and stone in the residue will act as inert filler. The chemical analysis of a high-glass residue used in compounding test mixtures is given in table 1.
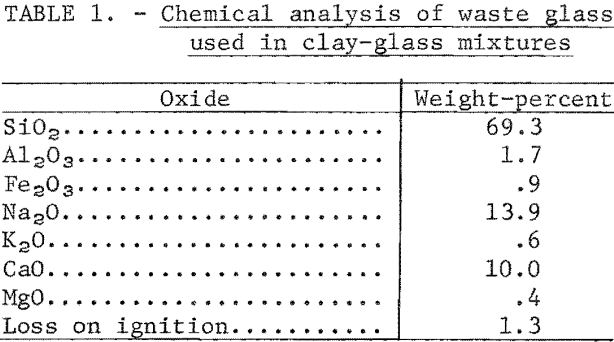
Bond Clays
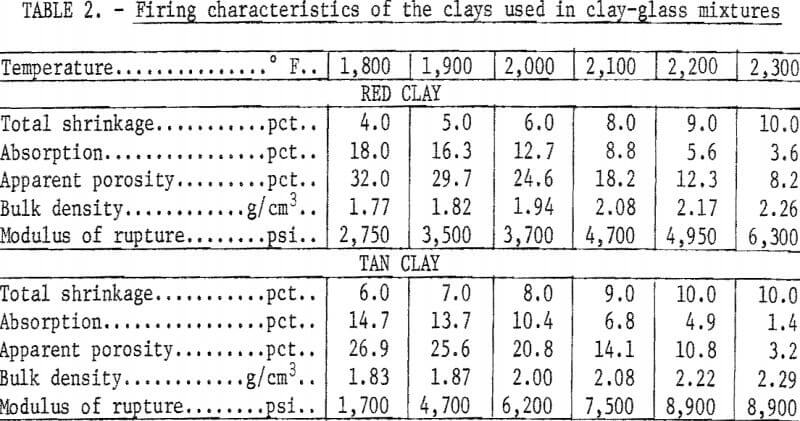
Tan clay-glass mixtures were prepared using a coal measure underclay from Walker County, Ala. This clay is a mixture of kaolinite, illite, a small amount of montmorillonite, and about 50 percent free quartz. The clay has fair bonding strength and fires to 6 percent absorption at about 2,125° F.
Red clay-glass mixtures were prepared using a clay from fiuvial deposits in Dallas County, Ala. This clay is also a mixture of kaolinite, illite, montmorillonite, quartz, and minor amounts of feldspar and limonite. The clay fires to 6 percent absorption at about 2,150° F.
The firing characteristics of the bond clays were determined using ½-inch-diameter rods formed by ram-press extrusion. The rods were fired to the indicated temperature at a rise of 100° F per hour and were soaked 1 hour at temperature. The physical properties of the fired clays are given in table 2.
Fluxing Tests
Glass Particle Size
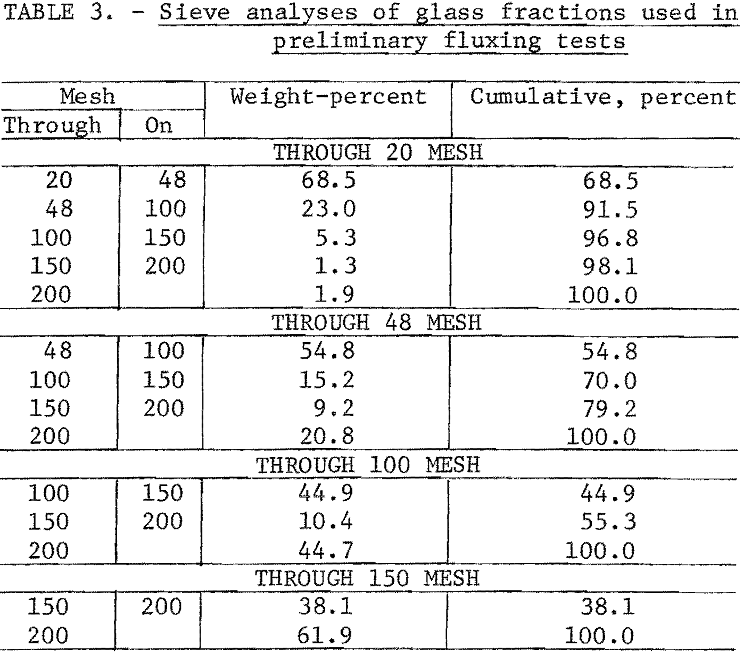
Preliminary fluxing tests were made to determine the optimum particle size for the glass fraction. The glass was stage-crushed to size using rolls. Sieve analyses of the size fractions tested are in table 3.
An arbitrary mixture of 60 percent minus 20-mesh tan clay and 40 percent glass was used in the preliminary fluxing tests. One- by 1- by 7-inch bars were formed using a laboratory de-airing extrusion machine. The bars were dried 24 hours in air followed by 24 hours at 212° F and were then fired to cone 08 (1,750° F) in an electrically heated kiln. Physical properties of the fired bars are given in table 4.
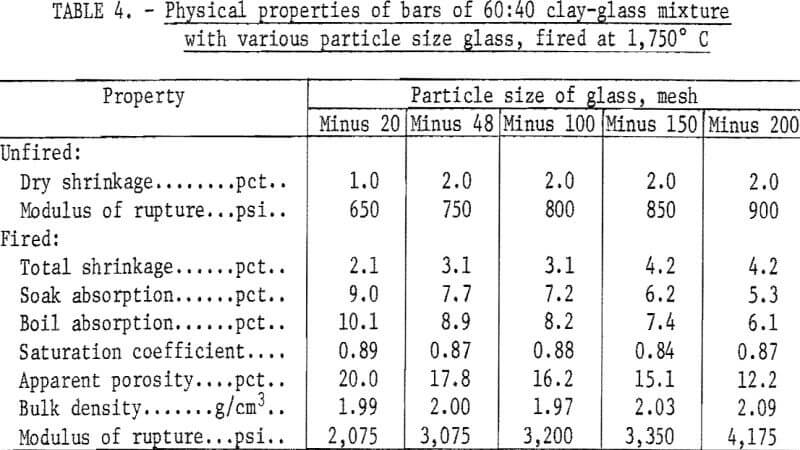
Results indicated that minus 200-mesh glass is the most effective flux; this size was used throughout the remainder of the investigation.
Clay-to-Glass Ratios
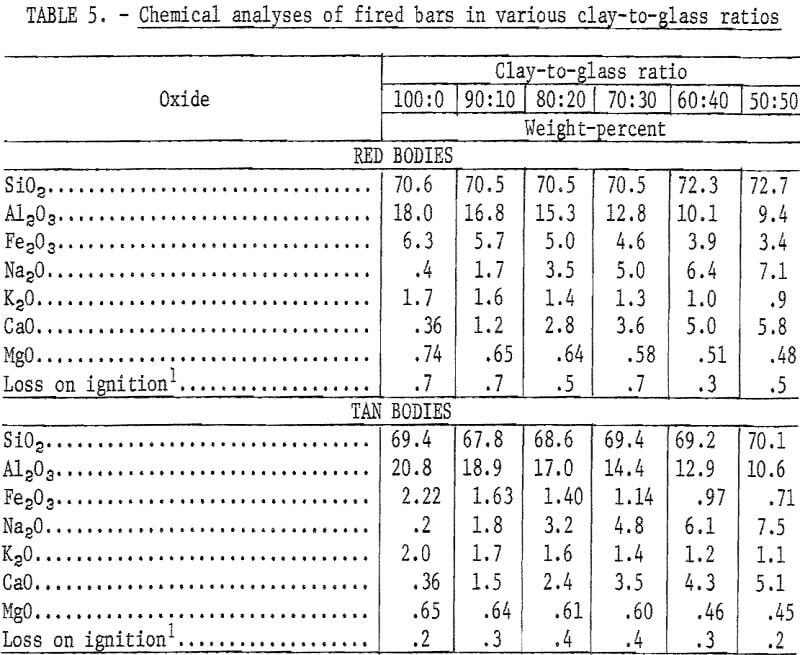
Mixtures of minus 20-mesh clay and minus 200-mesh glass in ratios of 100:0, 90:10, 80:20, 70:30, 60:40, and 50:50 were tempered with water in a dough mixer and then formed into 1- by 1- by 7-inch bars by stiff-mud extrusion. After drying at 212° F, 20 bars of each composition were fired to the required maturing temperature at a rise of 100° F per hour. The test bodies were considered mature when fired to 6 to 7 percent absorption, a range common to a variety of structural clay products including face brick, sewer pipe, and tile.
Chemical analyses of the mixtures tested are presented in table 5, and physical properties of the fired bars are presented in table 6. Previously reported research showed that brick containing in excess of 50 percent waste glass met specifications of the American Society for Testing and Materials.
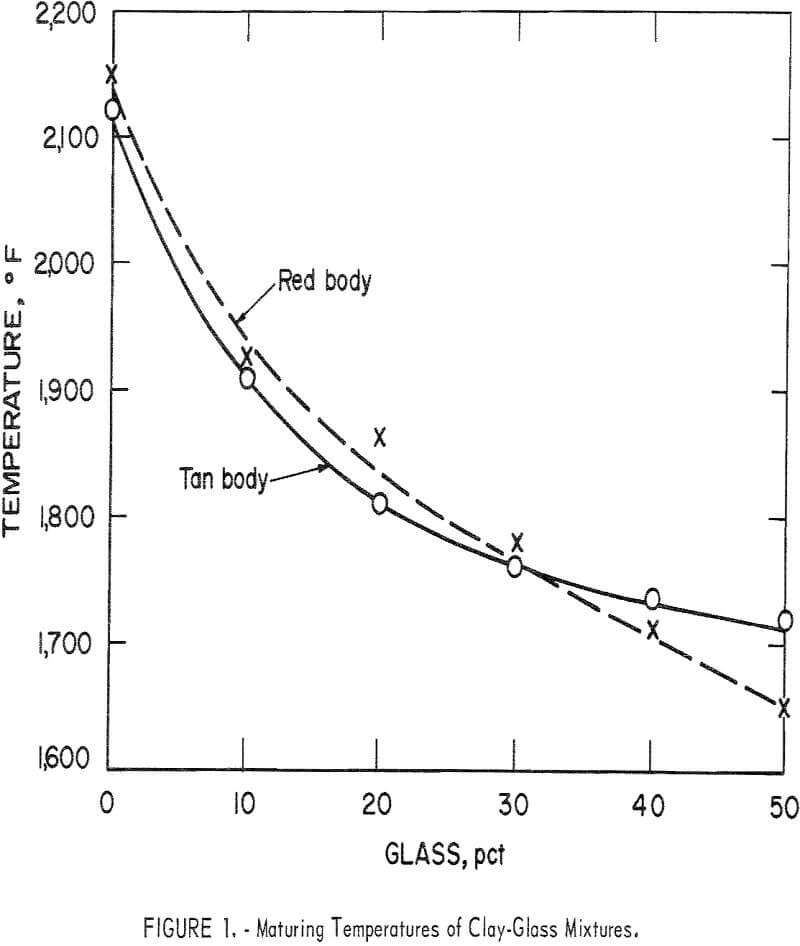
Variations in the silica content of the clay-glass mixtures were slight; the principal difference in composition was the increase of soda at the expense of alumina as the ratio of glass to clay increased. This was reflected by lower maturing temperatures (table 6 and fig. 1). The curves for the red and the tan clay-glass bodies were similar, although the curve for the tan mixture tended to level off more rapidly with substitutions of 40 and 50 percent glass for clay.
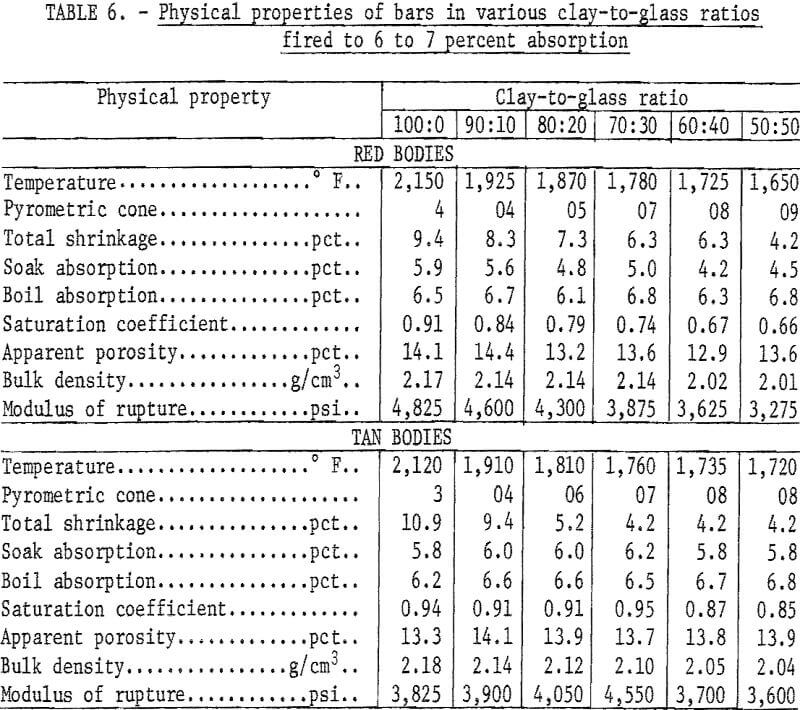
Nepheline (Na,K) (AlSiO4) was formed to some extent in all fired mixtures ; generally, the amount of nepheline increased as the glass-to-clay ratio and the firing temperature increased. This could explain the drop in saturation coefficient, bulk density, and modulus of rupture of the clay-glass bodies as the glass content increased. The drop in cross-breaking strength is not of serious moment, and lowering the saturation coefficient can be considered advantageous inasmuch as structural products that have saturation coefficients greater than 0.88 would be suspect in weathering resistance.
Laminations were serious in both 100 percent clay bodies, but as the glass-to-clay ratios increased, the tendency to laminate lessened, and no imperfections were visible in the 50:50 clay-glass mixtures.
Heat Requirements
The heat required to raise 1 ton of each of the clay-glass mixtures to the maturing temperature is given in table 7. The calculation for the red 50:50 clay-glass mixture is as follows:
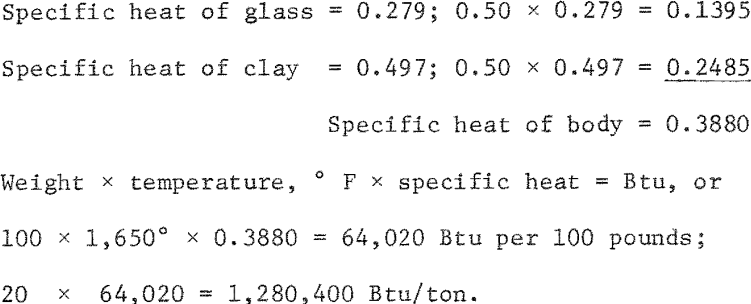
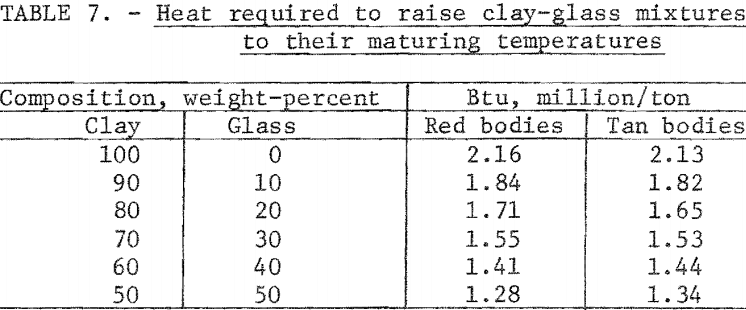
Substituting waste glass for one-half of the clay in red mixtures reduced the heat required to fire the body to maturity by 0.88 million Btu per ton; for the tan bodies the reduction was 0.79 million Btu per ton.
A plant that produces 200 tons of red ware per day would conserve 64,240 thousand cubic feet of 1,000 Btu gas per year; one that produces 200 tons of tan ware per day would conserve 57,670 thousand cubic feet. This represents a significant saving in fuel cost that should more than cover the cost for sizing the waste glass to minus 200-mesh.
Firing Time
Because of their lower maturing temperatures, the clay-glass mixtures can be fired in less time than can the 100 percent clay body, and the possibility of increased production with no added capital expenditure is perhaps the greatest benefit that might accrue from the substitution of waste glass for a portion of the clay in structural products. An example, based on tunnel kiln firing at a rise of 100° F per hour to temperature, follows:
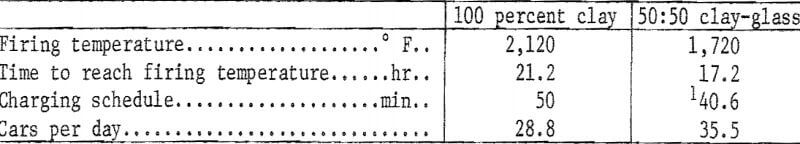
The potential increase in production using a 50:50 clay-glass mixture amounts to 23.3 percent. In a plant that produces 36 million face brick per year, the increase would amount to 8.388 million brick; at an f.o.b. price of $50.00 per thousand, the added production would increase plant sales by $419,400.
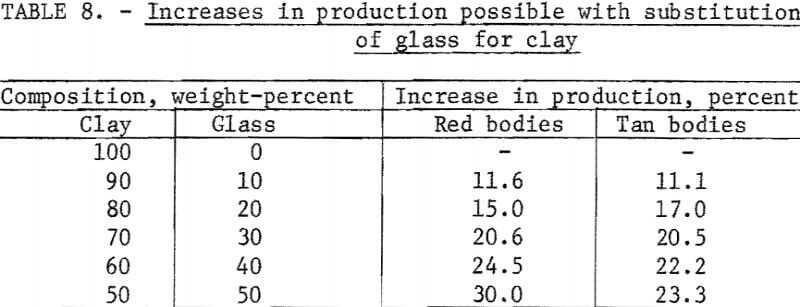
The potential increases in production for each clay-to-glass ratio are given in table 8.
Results of the investigation indicate that waste glass is an effective flux for common clays. Although nepheline was formed to some extent in all clay-glass mixtures, the effects were not considered of serious consequence.
Substitution of waste glass for one-half of the clay in a red body reduced the maturing temperature from 2,150° to 1,650° F, a reduction that could result in the conservation of 64,240 thousand cubic feet of 1,000 Btu natural gas per year and a 30-percent increase in production without additional kiln capacity.
Substitution of waste glass for one-half the clay in a tan body reduced the maturing temperature from 2,120° to 1,720° F, a reduction that could result in the conservation of 57,670 thousand cubic feet of 1,000 Btu natural gas per year and a 23-percent increase in production without additional kiln capacity.
