Table of Contents
According to Katz and Rabinowitch, workers do not agree as to the solubility of uranium tetrafluoride. However, the conditions under which solubility data were obtained were not always the same. In any event, since both uranium (III) and uranium (IV) fluorides are generally regarded as insoluble in a carbonate-free, nonoxidizing acid solution, in which fluoride ion complexing agents are absent, it seemed likely that uranium could be effectively separated from iron and certain other interferring ions by precipitation of the reduced solution with an excess of hydrofluoric acid.
This investigation indicated that, by using a double precipitation procedure, essentially quantitative separation of uranium tetrafluoride is possible for concentrations as low as 0.1 to 0.2 mg. of uranium per 100 ml. of solution, provided a suitable carrier is present and ammonium chloride is added to prevent peptization.
Preparation of Solutions
Uranium Solution
The stock solution, containing approximately 5 mg. U/ml., was prepared by fuming 2.948 g, of U3O8 (prepared by ignition of ammonium diuranate) with 10 to 15 ml. of HClO4 (72 percent) until decomposition appeared complete, adding water, filtering the solution, adding 25 ml. of HClO4 (72 percent), and diluting to 500 ml. in a volumetric flask. The standard solution was prepared by diluting the stock solution 1 to 4 with distilled water and enough HClO4 to maintain the original acid concentration. This solution was standardized against standard eerie sulfate, using the lead reductor procedure of Sill and Peterson.
Ceric Sulfate Solution
Cerium oxide (CeO2) was prepared by igniting the precipitate formed by the addition of oxalic acid to a hot solution containing 30 g. of (NH4)2Ce(NO3)6·2H2O in 200 ml. of water. To prepare 8 l. of approximately 0.005 N solution, 7.23 g. of the ignited CeO2 was taken up in sulfuric acid (1 to 1), diluted, and filtered through a fritted glass funnel. Concentrated sulfuric acid was added so that the total volume of acid was 213 ml. The solution was diluted to 8 l. with distilled water and stored in the dark in a glass bottle. After standing, the solution was standardized against arsenic trioxide in the presence of osmic acid according to the directions of Gleu and also against a standard uranium solution, using the lead reductor method of Sill and Peterson.
All other solutions were prepared from c.p. or reagent-grade chemicals.
Preparation and Use of Reductors
Lead Reductor
The lead reductor was prepared according to the directions of Sill and Peterson, except that 20- to 80-mesh lead and a smaller column (1 by 14 cm.) were used.
To reduce the uranium solutions, 8 ml. of concentrated hydrochloric acid and enough water to give a total volume of 35 ml, were added to the uranium solution (sulfate or perchlorate). The solution was passed through the reductor at a rate of 10 to 20 ml. per minute and the column washed with four 9-ml, portions of 1 N HCl.
Jones Reductor
The zinc amalgam was prepared according to the directions of Kolthoff and Sandell, using a 50-ml, burette (I.D. about 1 cm.). The height of the reducing column was 20 cm. A small plug of glass wool was inserted at the top and bottom of the column. (To prevent clogging of the lower glass-wool plug, it was found advantageous to use a small perforated porcelain disk at the bottom of the column before inserting the glass wool.) The column was washed thoroughly with water and kept covered with water when not in use.
To reduce the uranium solution (sulfate or perchlorate) containing about 2 ml. of concentrated sulfuric acid, it was diluted to 35 ml. with water, passed through the reductor at a slow rate (about 10 ml. per minute) and washed with four 9-ml. portions of 1 to 20 sulfuric acid. Solutions with high iron contents were partly reduced with 30-mesh zinc (about 1 g.) before reduction in the column.
Application of Fisher Titrimeter
Although other workers have used potentiometric methods to determine the end point in titrating reduced uranium solutions with ceric sulfate, the Bureau of Mines considered it desirable to test the suitability of an instrument, such as the Fisher titrimeter, for possible use in routine uranium determinations.
To check the accuracy and precision of the titrimeter end points, samples containing 10, 20, and 50 mg. of uranium were reduced in the lead reductor and collected in 10-ml. portions of 5 percent ferric sulfate. The volume was made up to 250 ml. with 1 N HCl and 50-ml. aliquots titrated with ceric sulfate solution. The instrument was calibrated at a scale setting of 1.00. A 10-ml. burette, graduated to 0.02 ml., was used to deliver the titrant. Titration curves were plotted and the end points compared with those obtained for the same solution by the “magic eye” of the instrument with a scale setting of minus-0.60 v. At least two titrations were made in each case. Good agreement was obtained between titration curves and magic-eye end points. The results are given in table 1.
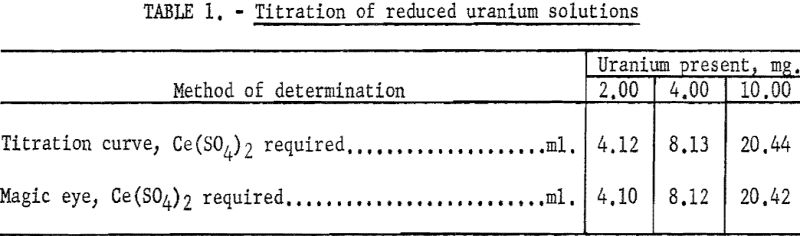
In checking the accuracy of the potentiometric titration, reduced solutions, containing 1.016 and 0.500 mg. of uranium, were titrated with standard ceric sulfate, the end points being detected by the magic eye. Recoveries of 99.6 and 98.2 percent, respectively, which are within the titration error, were obtained. However, for concentrations as low as 0.1 mg. of uranium, the results were not satisfactory, and it was concluded that the lower limits of accuracy lie between the range of 0.1 to 0.5 mg, of uranium.
Separation By Hf Precipitation
Results of preliminary tests indicated that more complete separations were possible if a soluble salt was added to suppress peptization of the uranium fluoride precipitate and a suitable carrier was present. It was found that essentially quantitative separations were obtained for samples containing a few tenths of a milligram of uranium in the presence of high concentrations of iron (250 to 500 mg.) in 75 ml. of solution by the addition of 2 g. of ammonium chloride and 20 to 50 mg. of lanthanum as the perchlorate.
Preliminary data also indicated that a double precipitation would be necessary to achieve quantitative separation of the fluoride. To carry out a double precipitation, it was desirable to use a reagent that would dissolve the first fluoride precipitate without oxidation of the uranium. According to Katz and Rabinowitch, a number of such reagents are available. After preliminary tests were made with some of these reagents, phosphoric acid was found to be best suited for this purpose. The general procedure found to be most satisfactory is outlined as follows:
Double HF Precipitation
To the uranium solution (about 75 ml.), reduced in the Jones reductor, 2 g. of ammonium chloride, 2 ml. of lanthanum perchlorate (20 mg. La), and 20 ml. of hydrofluoric acid (50 percent) were added. The mixture was transferred quantitatively to Pyrex centrifuge tubes and centrifuged at about 3,000 r.p.m. After the supernatant liquid was discarded, the precipitate was washed twice with 10 to 15-ml. portions of 2 percent HF-2 percent NH4Cl solution and dissolved by stirring and boiling with 2 to 4 ml. of phosphoric acid (85 percent). The resulting solution was cooled and diluted to 25 ml. with 1 N HCl, and 0.6 g. of ammonium chloride and 10 ml. of hydrofluoric acid (50 percent) were added. The solution was stirred, centrifuged, and washed twice with HF-NH4Cl solution as in the first precipitation.
Dissolution and Final Reduction
The fluoride precipitate was dissolved in the centrifuge tube by fuming with 5 ml. of perchloric acid (72 percent). After cooling, 8 ml. of concentrated hydrochloric acid was added and the solution diluted to 35 ml. with water. The cooled solution was reduced with standard eerie sulfate in the lead reductor just before titration in the manner described earlier.
Titration Procedure
After adding 0.5 ml. of 0.001 M ferroin and 1 drop of phosphoric acid to the reduced solution, it was titrated rapidly using a magnetic stirrer, to within 1 to 2 ml. of the end point or until the pink color was almost discharged. One milliliter of phosphoric acid (85 percent) was added, and the titration continued more slowly until the pink color (which was restored by the addition of phosphoric acid and more titrant) was discharged and the solution remained colorless for at least 20 to 30 seconds of stirring. Using 0.005 N Ce(SO4)2, a blank of about 0.16 ml. was usually obtained.
Discussion of Results
Although preliminary work with the Fisher titrimeter indicated that the results obtainable compared favorably with those for the visual method used by Sill and Peterson, it was concluded that any possible merits of the potentiometric over the visual method of titration are negligible, if not outweighed, especially in samples containing 1 mg. or less of uranium, because of the time lag in attaining equilibrium at or near the end point for such low concentrations.
Consequently, the visual method was used in all subsequent work. The titration procedure was essentially that of Sill and Peterson, with certain modifications because of reduced volume of solution, reductor size, and concentration of titrant. All titrations were carried out at room temperature.
Several tests were made to determine the accuracy and precision of the double HF precipitation procedure, as well as the effect of certain modifications in this method. The results are given in table 2.
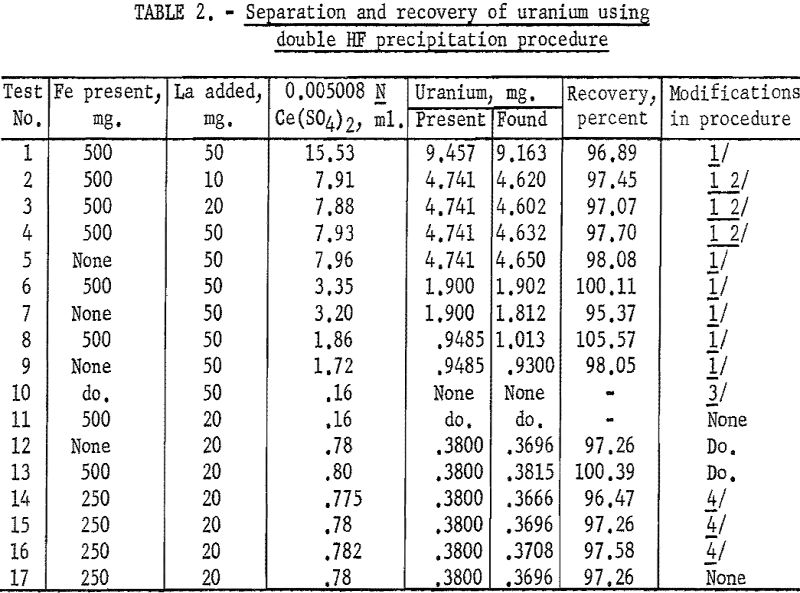
Analysis of the results presented in table 2 showed that iron was effectively eliminated by the double HF precipitation procedure for quantities as high as 500 mg. Slightly high results in tests 6 and 8, containing lower concentrations of uranium, indicated that a single washing of the second fluoride precipitate did not effectively remove traces of iron. This was further evidenced by tests 10 through 17, in which the precipitate was washed twice. Lower recoveries were obtained in tests 14 and 15 in which less than 15 ml. of hydrofluoric acid per 100 ml. of solution was used. It will be noted that for tests 12, 13, and 17, in which the previously described procedure was followed without modifications, the average recovery was 98.3 percent for samples containing less than 0.4 mg. of uranium. This represents a loss of less than 0.006 mg.
Use of the method on samples containing still lower concentrations of uranium indicated that the lower limits for accuracy comparable to the above lie in the range of 0.2 to 0.3 mg. of uranium.
To determine the effectiveness of this procedure in removing other possible interferences besides iron, tests were made on synthetic samples containing 250 mg. Fe, 25 mg. each of Ca, Mg, Al, and V, and 5 mg. each of Cu, As, Sb, Sn, Cr, Mn, Mo, Ti, and Nb, as well as known amounts of uranium. The results were erratic and indicated that this method, though effective for the separation of iron, would not be reliable for so complex a mixture without employing other methods of separation. The results were all high, except for samples in which iron or tin or both were absent. No attempt was made to establish the cause of interference, since acceptable methods are available to remove these ions prior to precipitation with hydrofluoric acid.
Application of Method
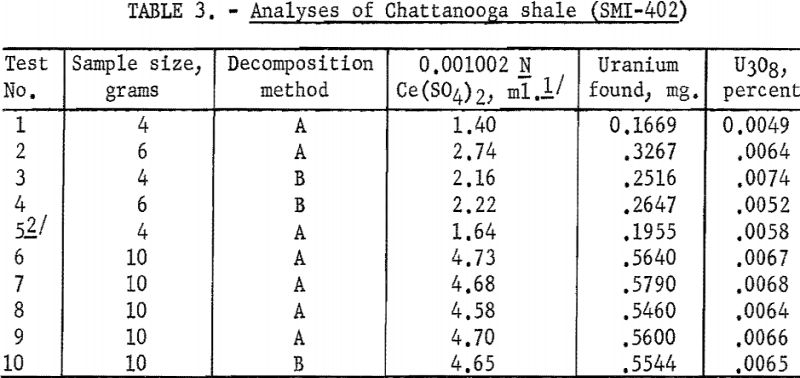
To test the application of the double HF precipitation procedure to a low-grade uranium sample, Chattanooga shale was selected, because, as stated earlier, the determination of uranium in this type of material was the primary purpose of this study. Since the uranium content of shale may run as low as only a few parts per million, it was considered desirable to use a sample of at least 10 g., although several tests were made with smaller samples.
Decomposition of Sample
After weighing, the samples were ignited 15 to 20 minutes to remove organic and volatile matter. They were then decomposed by one of the following methods, both of which gave satisfactory results.
Method A. – The ignited sample was treated with 10 to 15 ml. of hydrofluoric acid and twice evaporated to dryness on a steam bath. About 15 ml. of perchloric acid was added and fumed almost to dryness. The sides of the platinum dish were rinsed once or twice during the fuming period.
Method B. – This method was the same as A, except that the hydrofluoric acid treatment was omitted.
Separations and Titration
The only preliminary separation considered advantageous in the analysis of this particular sample of shale was the sulfide precipitation in acid solution. The directions given by Sill and Peterson, using thioacetamide, were followed, except that sulfurous acid was used as the preliminary reducing agent. After removing and washing the sulfide precipitate, the filtrate was reduced in volume to about 35 ml, prior to reduction in the Jones reductor as described earlier. The double fluoride precipitation was carried out and the samples titrated in the usual manner with ceric sulfate, except that a more dilute solution of titrant and 0.3 ml. of 0.001 M Ferroin were used.
Results given in table 3 indicate that for the smaller samples (4 to 6 g.), in which the uranium content was only 0.2 to 0.3 mg., the agreement was not as good as for the larger 10-g. samples, thus corroborating an earlier observation as to the lower limits of reasonable accuracy for the method. However, for the larger samples, the agreement was quite close (±0.0002 percent or ±2 p.p.m.).
As a further check on the effect of sample size, samples of an ore from Camp Creek, Idaho, which contained approximately 70 percent magnetite and about 10 percent ilmenite, were analyzed for uranium according to the previously described procedure. Results of 0.0226 and 0.0224 percent U3O8, respectively, were obtained for 2- and 4-g. samples.
Chemical Symbols Used in this Presentation
To assist certain readers, the following explains the chemical symbols used in this publication: Fe2O3 (ferric oxide); U3O8 (uranium oxide); HClO4 (perchloric acid); CeO2 (cerium oxide); (NH4)2Ce(NO3)6·2H2O (ammonium cerium nitrate); HCl (hydrochloric acid); Ce(SO4)2 (ceric sulfate); La (lanthanum); HF (hydrofluoric acid); NH4Cl (ammonium chloride); Fe (iron); Ca (calcium); Mg (magnesium); Al (aluminum); V (vanadium); Cu (copper); As (arsenic); Sb (antimony); Cr (chromium); Mn (manganese); Mo (molybdenum); Ti (titanium); Nb (columbium).
