The latest revised edition of Mr. C. A. Stetefeldt’s book on the Lixiviation of Silver-Ores, which appeared very recently, contains no mention of the volatilization of silver in chloritization-roasting—an omission which is the more remarkable in view of the fact that in former editions of the work this important subject was noticed. Moreover, Mr. Stetefeldt has discussed it to some extent in his paper on “ The Stetefeldt Furnace,” in which he criticised certain statements made by me before the Colorado Scientific Society, in a paper entitled “ A Review of the Russell Process; ” and Mr. Morse, in his paper on “ The Lixiviation of Silver-Ores by the Russell Process at Aspen, Colorado,” has given statistics showing the loss of silver by volatilization (including the dust loss, which Mr. Morse estimates at not more than 1 per cent.) on more than 30,000 tons of ore roasted, to have been 9.16 per cent. The matter must certainly be deemed important enough to warrant me in returning to it, and offering, with the aid of the figures made public since my first paper, a reply to the criticisms of Mr. Stetefeldt.
The passages of my paper which he deemed to be “ questionable conclusions from a limited experience with the Stetefeldt furnace at the Holden mill, Aspen, Colorado,” were quoted by him, and are here repeated for the convenience of the reader:
” The use of this furnace has certain limits beyond which it is unwise to go. Siliceous ores carrying 6 per cent, or more sulphur can be chloridized better in some other furnace. Ores carrying a large percentage of lime—say from 15 to 20 per cent, of CaO—are also very difficult to chloridize properly in the Stetefeldt furnace, unless sufficient sulphur is present to combine with the CaO and form CaSO4; even then the chloritization in the furnace is frequently very low, and rarely exceeds 60 per cent.
“ The writer has seen the furnace deliver roasted ore from certain mixtures high in lime and sulphur where not more than 15 to 20 per cent, of the silver had been chloridized in the shaft of the furnace, where about 65 per cent, of the ore is roasted. However, after the above ore had been lying on the cooling-floor for three days, fully 90 per cent, of the silver was found to be chloridized. The extremely low percentage of the silver converted into chloride in the furnace was due partly to its having been crowded beyond its capacity, but chiefly to the large percentage of sulphur present causing such a strong reducing atmosphere of sulphur dioxide that the effect of the chlorine liberated by the acid gases was neutralized. Frequently the odor of sulphurous acid escaping from the roasted ore as it was discharged from the shaft of the furnace was sufficiently strong to overcome all smell of chloride fumes. The sulphur in the raw charge of the ore just considered ranged from 8 to 10 per cent, being about half that of the lime and magnesia present.”
“ Can such ores be roasted better with any other furnace ? Metallurgically, the answer is, Yes. The proof of the above assertion with regard to Aspen ores has been demonstrated by the writer by roasting in a reverberatory furnace 10 lots of ore containing 25 per cent of CaO (MgO not determined, but probably amounting to 10 or 12 per cent.) with less than 2 per cent, of sulphur, and using, practically, the same amount of salt as in the Stetefeldt furnace. The chloridization was all that could be desired.”
“ For ores containing from 3 to 8 per cent, of sulphur, the Bruckner, Pearce or Howell-White are to be recommended.”
To these statements Mr. Stetefeldt makes two general objections : first, that they were based on too limited an experience ; second, that they were not accompanied with adequate data in the way of proof. As to the first, I venture to submit that while my experience was confessedly limited to the ores of a certain district, it comprised nearly a year of continuous observation, practice and experiment, and was, therefore, quite long enough to warrant the formation of an opinion concerning the materials to which it referred, and, by analogy, other similar materials. As to the second objection, I have only to say that, at the time I wrote the Aspen works were still in operation, and the company was unwilling to have exact details made public. This deficiency has been completely made up through the later publication of complete statistics by Mr. Morse in his paper, above cited.
Mr. Stetefeldt points out what seems to him an inconsistency between my statement that “ siliceous ores carrying 6 per cent, or more sulphur can be chloridized better in some other furnace ” than the Stetefeldt, and my subsequent statement that “ for ores containing 3 to 8 per cent, of sulphur, the Bruckner, Pearce, or Howell-White are to be recommended.” But he will perceive, on reflection, that the two propositions are not inconsistent. The hypothesis, for instance, that ores carrying from 3 to 6 per cent, of sulphur could be chloridized as thoroughly by the Stetefeldt as by either of the three rival furnaces named, but that, for such ores, one of the three was to be recommended on other grounds, such as smaller cost of construction, or smaller loss in roasting, would remove the fancied contradiction.
Leaving these preliminary and subordinate criticisms, let us consider the first important point urged in opposition to my conclusions, namely, that the Aspen ore is exceptional and peculiar, and must be acknowledged to be “ a difficult ore to chloridize in the Stetefeldt as well as in any other furnace.” This difficulty in a Stetefeldt furnace is admitted by all who have made the attempt to overcome it; but the proposition cannot be admitted as regards “ any other furnace.”
On page 103 of Mr. Stetefeldt’s book is given a description of the manner in which an ore should be tested to determine its fitness for lixiviation. It is there observed, that roasting- tests are best carried out in the muffle of an assayer’s cupelling-furnace, in clay dishes about 4½ to 5 inches in diameter, holding a charge of 3½ A. T. On the following page we read:
“It is not always possible to produce by muffle-roasting, on a small scale, the same effect that can be obtained by actual mill-work; especially if an ore is treated requiring banking up on the cooling-floor for many hours, in order to reach a high chlorination of the silver. Hence, an unfavorable result is not always a proof that the ore is unsuitable for lixiviation.”
The natural conclusion is, that if an ore is easily chloridized in small quantity, in an assay-furnace, it would be as well, or better, chloridized on a commercial scale in a large furnace. As to the correctness of this proposition, I will say nothing here; but, accepting it for the present as true, I give the results of its application to the Aspen ores.
The experiments, the results of which are recorded below, were made as follows: The raw ore was crushed so as to pass through an 80-mesh sieve, 150 grammes taken and thoroughly mixed with 16 per cent, salt and 4 per cent, of iron pyrites containing about 40 per cent, of sulphur, roasted from 15 to 30 minutes, commencing with a very low and ending with a light- red heat. After cooling, the roasted mixture was carefully removed from the dish and weighed, and again assayed to determine the loss by volatilization, the percentage of soluble salts also being determined. Leaching-tests were now made to determine the percentage of silver-chloride formed in the roasting, and the proper method of applying the Russell-solution to obtain the highest possible extraction of the silver.

A great many experiments with results similar to the above might be recorded here. In fact, the results were all so uniformly good, that they soon became monotonous, and the experiments were discontinued.
In view of the above results, I claim that Mr. Stetefeldt is not warranted in making the statement that the Aspen ores are difficult to chloridize in any furnace. Mr. Stetefeldt says that the fact that the sulphur in the Aspen Ore (as mixed for roasting) exceeds the limit given by me from 2 to 7 per cent., would force one to the conclusion that the Stetefeldt furnace at the Holden mill was a complete failure, and that Mr. Morse’s statistics contradict this. Instead of contradicting, Mr. Morse has since confirmed this fact very conclusively.
Mr. Stetefeldt says:
“It is well known that high chlorinations of silver-ores containing a large percentage of pyritic minerals can only be obtained after roasting in a Stetefeldt furnace by leaving the discharged ore for twenty-four hours, or longer, in heaps on the cooling-floor. But why should this be made an objection?”
In my review of the Russell process, reasons were given for considering heap-roasting on the cooling-floor extremely objectionable. Mr. Morse, in his paper on “ The Effect of Washing with Water upon the Silver Chloride in Roasted Ore,” while differing with me as to the reactions involved, is equally emphatic in condemning this heap-roasting for ores sustaining as much zinc (from 1 to 3 per cent.) as those treated at Aspen. And Mr. Stetefeldt himself, on page 65 of the last edition of his book, accepting Mr. Morse’s theory as to the cause of the trouble, indirectly condemns heap-roasting and the Stetefeldt furnace for all ores containing from 1 to 3 per cent, of zinc, and a sufficient amount of pyritic minerals to make such supplementary heap-roasting necessary.
He controverts my statement that the supply of free oxygen is extremely limited in the heap on the cooling-floor. The reactions given by him as occurring in this heap-roasting are undoubtedly correct, but he does not show that these are the only ones which occur; and in any event, the fact that three or four days are required to oxidize by means of these reactions the comparatively small amount of sulphur still in the ore, seems to me conclusive evidence that the admission of air to the interior is very slow and limited. This impression is confirmed by the fact that the crust of the heap chloridizes in as many hours as days are required to chloridize the interior to an equal degree.
My theory as to the loss of silver by volatilization in chloridizing-roasting is declared to present nothing new. But the question of originality is not so important as the question of fact.
In the first edition of his book, Mr. Stetefeldt said:
“ It is a well-established fact that the loss of silver by volatilization in roasting in a Stetefeldt furnace is a minimum and almost imperceptible, this loss being principally a function of time.”
It was this statement which I controverted in the paper which he criticizes. What he now says is:
“The evaporation or volatilization of all substances is governed by the same general laws. The effective elements are: time, temperature, surface exposed, character of the atmosphere in which evaporation or volatilization takes place, density or pressure of the latter, and its motion or exchange in relation to the substance evaporated or volatilized. Thus, for instance, more silver is volatilized in roasting a small ore sample in a muffle than in actual reverberatory-furnace work, because more surface is exposed, and the particles have more contact with air in the former case.” (The italics are mine.)
In the paper criticized, I said on this point:
“ Mr. Stetefeldt claims that the volatilization of silver is principally a function of time. If the above statement is true, it is one of the strongest arguments in favor of the Stetefeldt furnace. Experiments made to determine this point proved that time was an important function under certain conditions, but not at all under all conditions. There is another factor which, so far as I know, has never been mentioned in connection with the loss of silver in chloridizing-roasting, which factor is air, or oxygen. That oxygen is one of the principal if not the principal cause of the volatilization of silver in chloridizing-roasting, is believed by the writer for the following reasons :
“It is possible to volatilize from 40 to 60 per cent, of the silver contents in an ore in a chloridizing roast conducted in a muffle at a low, red heat, in fifteen to thirty minutes. The same ore roasted at the same temperature with the same percentage of salt on a commercial scale, in a large furnace, under the worst conditions, would not show more than one-third of the loss sustained in the muffle, while under the best conditions the loss would, in all probability, not exceed one-tenth of that experienced in the muffle. The time required for roasting in any large furnace other than the Stetefeldt would be many times that required for the muffle roast. If it can be shown that, with only a momentary exposure of the ore, as is the case in the Stetefeldt furnace, the percentage of silver volatilized is as high, or nearly as high, as when the ore is roasted for eight hours with the same quantity of salt in a reverberatory furnace, it must be concluded that there are other influences more important than time governing such loss. This has been found to be the case with the Aspen ores. The average loss by volatilization determined in roasting some 20-ton lots of ore in a reverberatory was found to be less than that experienced at Aspen, where the Stetefeldt furnace is used.
“In the above cases the following conditions were the same: Character of ore, percentage of salt used and temperature of roasting. Those not the same were : Time of roasting and amount of oxygen in contact with the particles of ore during the time the chloridizing action was going on.
“ Time only increases the volatilization of silver when sufficient heat and air are present. The highest loss was sustained in the muffle where the amount of air used in roasting was greatest. The ore roasted in the reverberatory was just as well chloridized and gave fully as high extraction as that treated in the Stetefeldt furnace
“It was also noticed at Aspen that some of the lowest losses in silver were experienced during the months when a heavy excess of sulphur had been used and only a limited supply of air allowed to enter the furnace ; it was also afterwards observed that additional air produced a higher chloridization of the silver, but that the losses by volatilization were also higher. Every indication pointed to the fact that the higher chloridization in the furnace was obtained at the expense of part of the silver. The fact of a smaller loss by volatilization when roasting with a higher percentage of salt can only be explained on the same theory, namely, that the atmosphere enveloping the ore is one of chlorine rather than oxygen. During the strong chloridizing action on the cooling floor where the ore remains red-hot to within a few inches of the surface for two, and frequently three days, the loss by volatilization is not perceptible, and samples taken from different parts of the heap at different intervals show no variation in value. It is true that all fumes are condensed and prevented from escaping into the air by the cold crust of ore, and the volatilization might be prevented more by a mechanical than chemical condition.
“In chloridizing-roasting gold-ores, the theory equally holds so far as the agency of air is concerned. The writer has roasted gold-ores side by side in Bruckner and Howell-White furnaces with the same percentage of salt in each. One of the Howell-White furnaces was built in front of the other and the ores allowed to pass through both. Nearly all the sulphur was driven off in the first furnace. The salt was added to the hot ore as it was being fed from the first furnace into the second, where it was roasted for a little over an hour. In the Bruckner the salt was added to the raw ore and the charges roasted from eight to twelve hours. The volatilization of gold was the heaviest in the Howell-White furnace, where undoubtedly each particle of ore was more frequently exposed and longer in contact with the air than in the Bruckner furnace. The same ore, when roasted in the muffle with the same percentage of salt at a low red heat for fifteen to thirty minutes showed nearly ten times as great a loss in gold by volatilization as was experienced in the practical operation.
“ It is evident from the above statements that determinations of the volatilization of silver and gold by chloridizing a few hundred grammes of ore in a muffle are not apt to be very reliable. According to Mr. Letts, Yedras ore is now being roasted (chloridized) in the reverberatory furnace at a loss of only 6½ to 7 per cent, of the silver.”
To meet the objection that the above argument was unaccompanied with detailed proof, I offer the following, which I trust will be convincing:

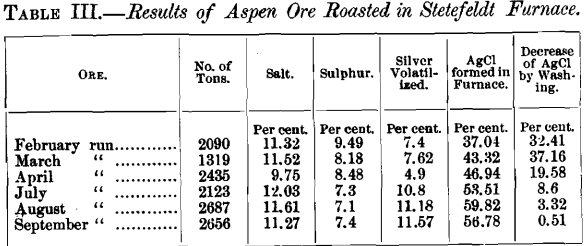
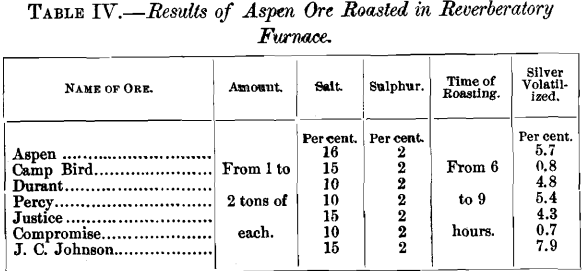
The above results need no comment, so far as the comparative volatilization of silver is concerned. Mr. Stetefeldt argues that the loss at Aspen might have been in dust escaping from the last dust-chamber to the chimney, and hence not fairly chargeable to volatilization. He cites the experience at the Ontario mill, where in flues about 150 feet long, 4 feet wide and 6 feet high, interposed between the dust-chambers and the chimney, 1.26 per cent, of the silver in the ore is recovered. It would be interesting to know the composition of the material saved in these flues, and especially the percentage of condensed fume containing silver previously volatilized. At Aspen, a considerable amount of such material was saved in the rear end of the dust-chamber, as may be seen from the following table:
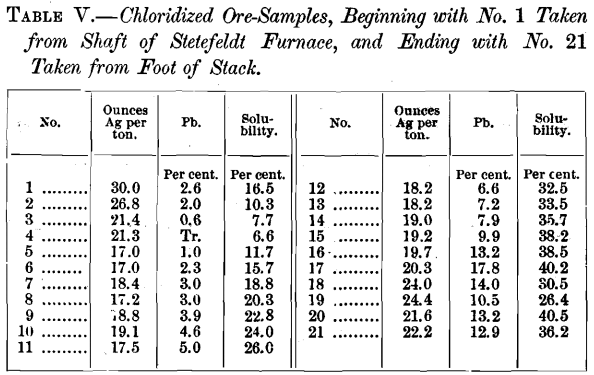
The actual volatilization of silver in the furnace itself, at Aspen was therefore even greater than the figures I have previously given, by the amount of volatilized silver thus recovered.
That the conditions above stated are not peculiar to Aspen ores exclusively, was indicated by the treatment of several hundred tons of Creede ores in Aspen furnaces. These ores contained approximately 90 per cent, of silica, no lime, and 1 per cent., or less, of sulphur. The detailed results are not in my possession; but I am informed that the chloridization in the furnace was remarkably good, but that the loss by volatilization was even greater than on any of the Aspen ores.
Mr. Stetefeldt concludes his paper with the frank admission that “ the fact that the loss of silver by volatilization during chloridizing-roasting in the Stetefeldt furnace is a minimum, under all circumstances, as compared with roasting in any other furnace, lacks absolute proof.” In view of the foregoing discussion, I think we may fairly ask for evidence that such loss in that furnace is a minimum under any circumstances.
Since this paper was put in type, the writer has received the news of the sudden death of Mr. Stetefeldt. If he had known earlier of this event, while, of course, it would not have altered the opinions and arguments stated by him, it would certainly have led him to express his cordial sense of the professional ability of Mr. Stetefeldt, the value of his work, and the loss inflicted upon the Institute by his death.
