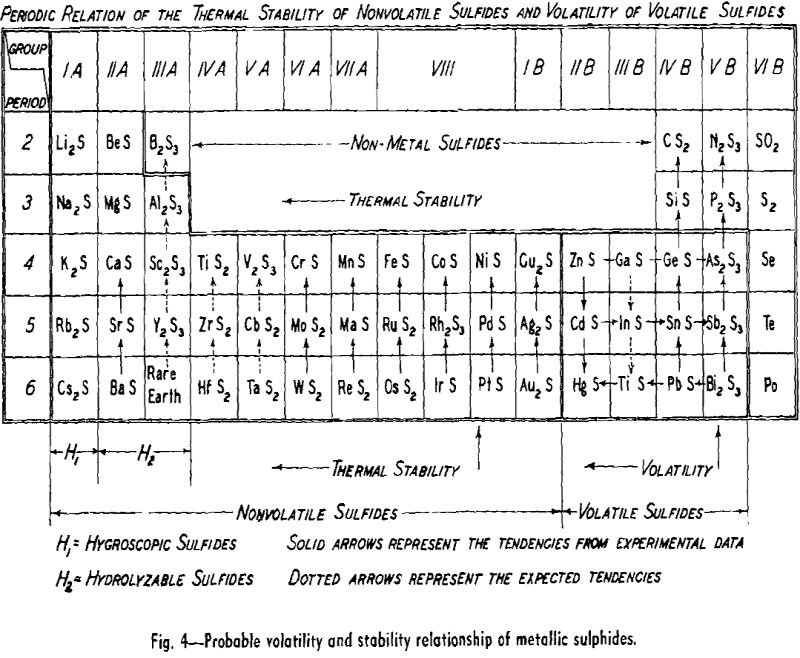
The direct reduction of metallic sulphides, as in the precipitation method for lead or antimony, is not a widely used process today. However, it might be employed for the production of a number of metals if the operation were carried out under reduced pressures. Two general procedures are possible; the reducing agent could form a stable non-volatile sulphide from which the valuable metal could be removed by volatilization or liquation. The other approach would be to choose a reducing agent which would form a sulphide that could be volatilized away from the valuable metal. A vacuum operation would serve to protect the sulphides and metals from oxidation and would increase the evaporation rates.
The experiments described in this paper consisted of determinations of the rate of weight loss of certain metallic sulphides under very low pressures at different temperatures. These data plus observation and analyses of the products reveal the mechanism of the weight loss and also provide a basis for the calculation of the vapor pressure or apparent vapor pressures of the sulphides.
Langmuir determined that the maximum rate of evaporation of substances of low volatility when heated in a vacuum is related to the vapor pressure as follows:
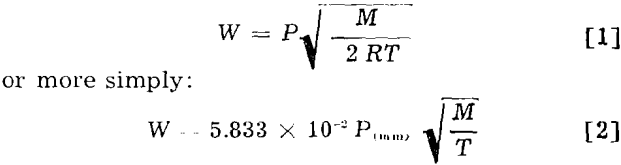
where W is the maximum rate of evaporation in g per sq cm per sec; P, the vapor pressure in mm of mercury; M, the molecular weight in g; R, the gas constant; and T, the absolute temperature.
To correct for the weight loss during the heating and cooling time, a second run was made with the same heating and cooling rate but a different holding time at the same temperature. The difference in weight loss between the two runs was then the result of volatilization at the holding temperature for the time equal to the difference between the two runs. Expressed in equation form:
G = (W1 – W2)/(t1 – t2)
where G is the rate of evaporation in g per sec; W1 and W2 the weight losses of runs 1 and 2; and t1 and t2 the total times of those runs. If G is divided by
the area of the sulphide surface the rate of evaporation in g per sq cm-sec is obtained.
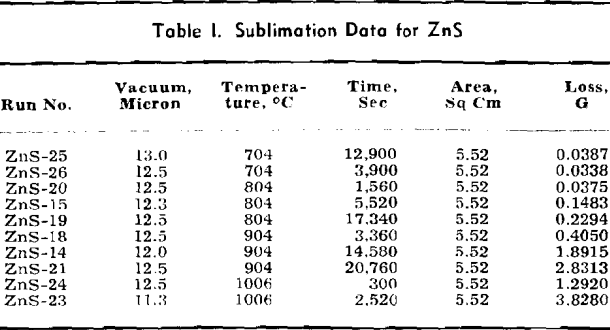
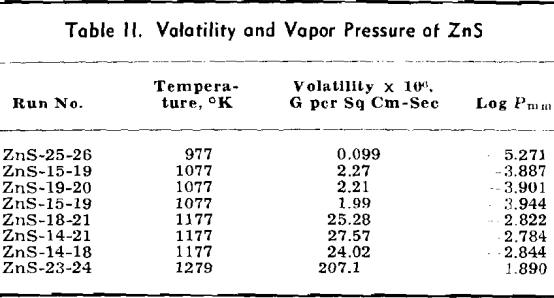
Volatile Sulphides: The data for ZnS are listed in detail and the methods of calculation are shown. Only the final data are shown for other volatile sulphides which are treated in the same manner as given for ZnS.
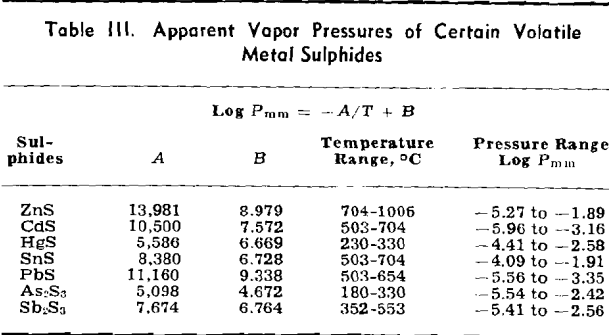
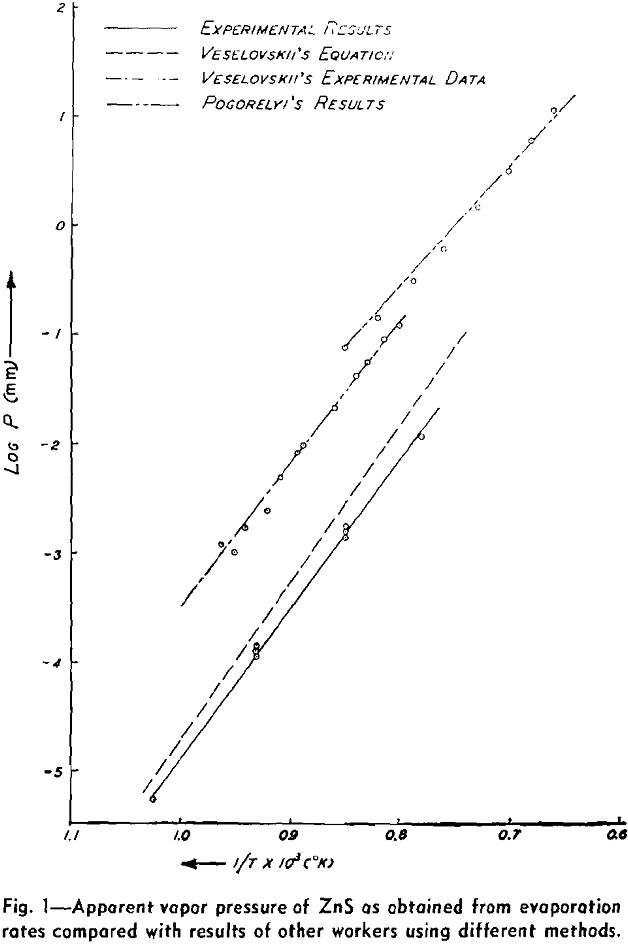
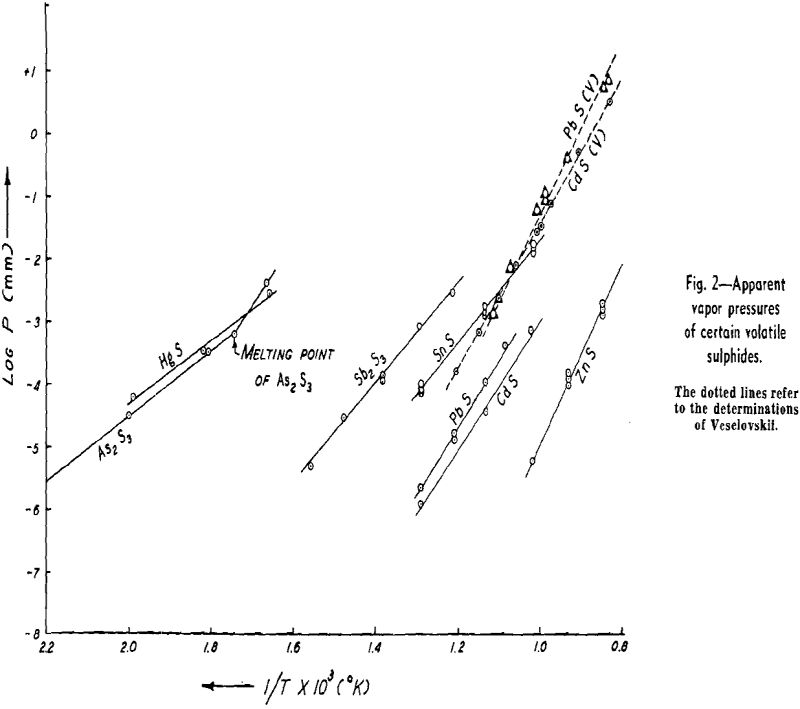
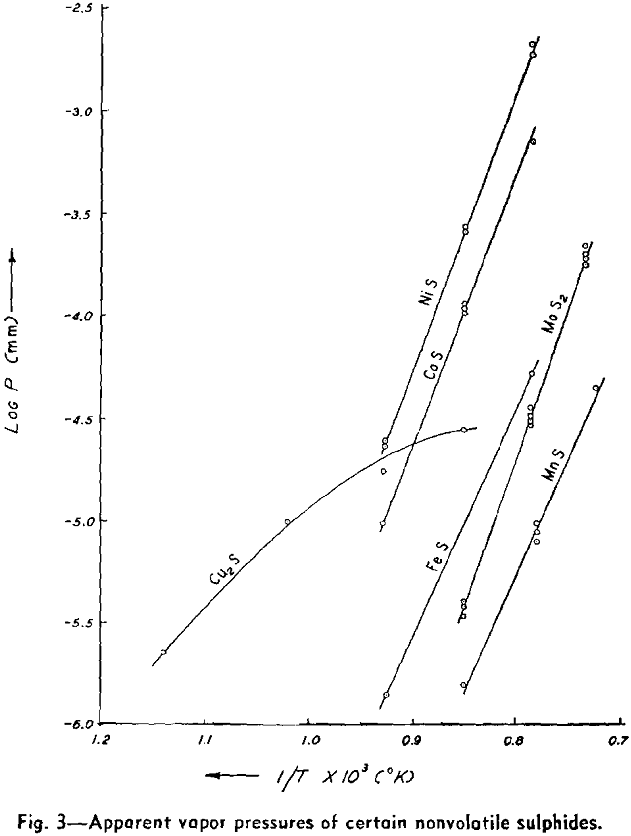
The weight looses for two or more runs at the same temperature are combined to eliminate the error of heating and cooling and the rate of loss is calculated. This rate is then substituted in Langmuir’s equation and the apparent vapor pressure is calculated. The data for log Pmm plotted against l/T gives a straight line. The equation of the line is:
log Pmm = 13,981/T + 8.979
Some doubt exists as to the behavior of As2S3. There is a possibility that it decomposes to As2S2 and sulphur, both of which volatilize, although there
was no free sulphur in the condenser to substantiate this theory.
NiS: The apparent vapor pressure is almost the same as the decomposition pressure reported by Jellinek and Zakowski. Chemical analyses indicate that the weight loss is at least 90 pct due to dissociation. Hayward has reported that NiS readily loses sulphur upon heating until the residue attains the relatively stable composition of Ni3S2.
Cu2S: The apparent vapor pressure of Cu2S does not follow the pattern of the other sulphides. The weight loss, especially at lower temperatures, is much greater than that expected on the basis of dissociation pressures determined by other workers by a factor of 1000. However, the formation of fine metallic copper wool on the surface of the sample is evidence of dissociation.