Addition of vanadium to iron and steel gives improved product properties such as increased strength, improved machinability, reduced distortion, simpler heat treatment, better weldability, increased wear resistance, better control and uniformity of hardness penetration and gradient, smoother and better finishes, and reduced flaking or spalling of carburized surfaces. Consequently, vanadium-steel alloys are used in the manufacture of products such as tool steels, structural members, mining equipment, airframes, and heads and cylinder linings of large diesel engines.
The United States had a vanadium net import reliance of 28 pct of apparent consumption during 1980. Of the total import supply, 58 pct is obtained from South Africa, 16 pct from Chile, 7 pct from Canada, and the remaining 19 pct from other nations. Because of its importance in manufacturing steel alloys and because of partial dependence on imports for adequate supplies, vanadium is listed as a strategic and critical mineral.
Domestic production of vanadium oxide during 1980 was estimated at 5,506 short tons. Over half of this material was produced from carnotite resources in the Colorado Plateau area. Typical flowsheets for this recovery have been reported by Merritt. The remaining vanadium oxide was produced from the Union Carbide vanadium mine at Wilson Springs, Ark., and as co-products from iron and phosphate operations in the West and Great Lakes area.
Additional vanadium resources have been cited such as phosphoria shales found in Idaho and Wyoming, dolomite shales found in Nevada, and metalliferous oil shales in central Montana and north-eastern Nevada. Another available supply may be found in low-grade carnotite deposits in the Colorado Plateau. None of these deposits are presently utilized for vanadium recovery, and investigations for treating these low-grade ores have not been reported. Greater utilization of these assets would broaden the domestic resource base of the Nation. This report examines several extraction techniques for vanadium and uranium from a current mill-grade and a low-grade carnotite ore sample. The mill-grade sample contained 1.26 pct V2O5 and 0.185 pct U3O8, and the low-grade ore sample contained 0.34 pct V2O5 and 0.039 pct U3O8. The investigation included the effects produced by several leaching parameters: leaching time, temperature, and various acid and oxidizing reagent concentrations.
Description of Ore Samples
Experimental comparisons were made using two carnotite samples obtained from the Colorado Plateau. Sample 1 was comparable to a present uranium mill-grade feed. Sample 2 was considered a low-grade material. The chemical analysis of each sample is presented in table 1.
Microscopic examination of sample 1 showed a fine-to-medium-grained sandstone. Quartz was the dominant mineral,
and many grains had secondary silica overgrowth. The presence of clay in some cases resulted from the weathering of feldspar grains. A black organic material was apparent in the sample, and minor amounts of calcite and pyrite were associated with this material. A small amount of carnotite was present on the surface of the quartz grains or partially filling the interstices. The carnotite was not distributed evenly throughout the sample.
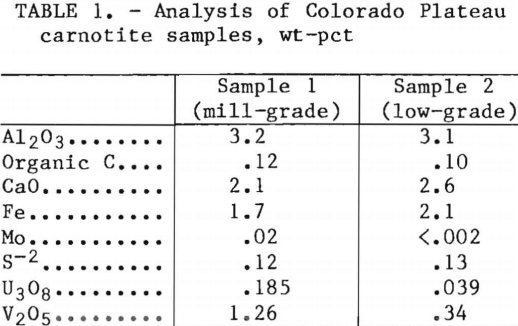
Sample 2 was also a sandstone and was very similar to sample 1, but the organic material appeared to be less abundant. Carnotite crystals were slightly more apparent in sample 2 than in sample 1. Figure 1 is a photomicrograph of mono-clinic tabular carnotite crystals found in the low-grade material.
Detectable amounts of vanadium were found in the black organic material in both samples. Although the vanadium- bearing phase could not be isolated, higher uranium and vanadium contents detected in sample 1 resulted from a higher amount of these elements within the black organic material.
Experimental Studies
Bench-scale experiments were performed to compare the extraction of vanadium and uranium from two ore samples with different concentrations of V2O5 and U3O8. The investigation evaluated various operating leach parameters and also compared the recovery of vanadium and uranium by acid agitation leaching, and autoclave acid leaching. Because H2SO4 is readily available and used commercially for leaching a mill-grade vanadium-uranium Colorado Plateau ore, it was selected for use in this investigation. Oxidizers used to promote the extraction of the metals included NaClO3 for the agitation leach tests and pressurized oxygen for the autoclave experiments. Although ore preroasting with NaCl would be an added expense and generally not advantageous, a comparison of this process with the other techniques was included.
Acid Agitation Leaching
Various comparative experiments were conducted using H2SO4 in agitation leach tests with 200-g ore samples. The samples, generally ground to minus 35- mesh Tyler screen size, were leached at a pulp density of 50 pct solids. Figure 2 illustrates a typical equipment arrangement for the experiments. Tests included examination of the following operating conditions: (1) reagent consumption, (2) leaching time, (3) temperature, and (4) ore particle size.
In addition, several tests were conducted to determine the effect of pugging with acid. The ore sample was pugged with an acid-water mixture to 20 pct moisture and baked for 18 hr at 110° C. The baked ore was water-leached at room temperature (23° C) for 4 hr at a pulp density of 50 pct solids.
When NaClO3 oxidant was used during an agitation-leach experiment, it was added to the slurry about 1 hr after the test started. This procedure prevented the premature consumption of the oxidant by gangue minerals. Once contact time had been concluded, the pregnant leach liquor was filtered from the solid residue on a Buchner vacuum funnel, and the solution pH and emf were measured. The filter cake was washed twice with a pH 1.5 solution and once with water. Pregnant filtrate and wash solutions were combined, and the total volume was measured prior to sampling. The emf values in millivolts were measured using platinum and saturated calomel electrodes. The more highly oxidized solutions were indicated by larger positive emf values. Mill target values might be a pH of less than 1.5 and an emf of 350 to 450. Leached solid residues were dried at 110° C, pulverized, and analyzed for vanadium and uranium. These analytical results were used to calculate the percent extractions and to prepare the metallurgical balances, which were generally within ±3 pct.
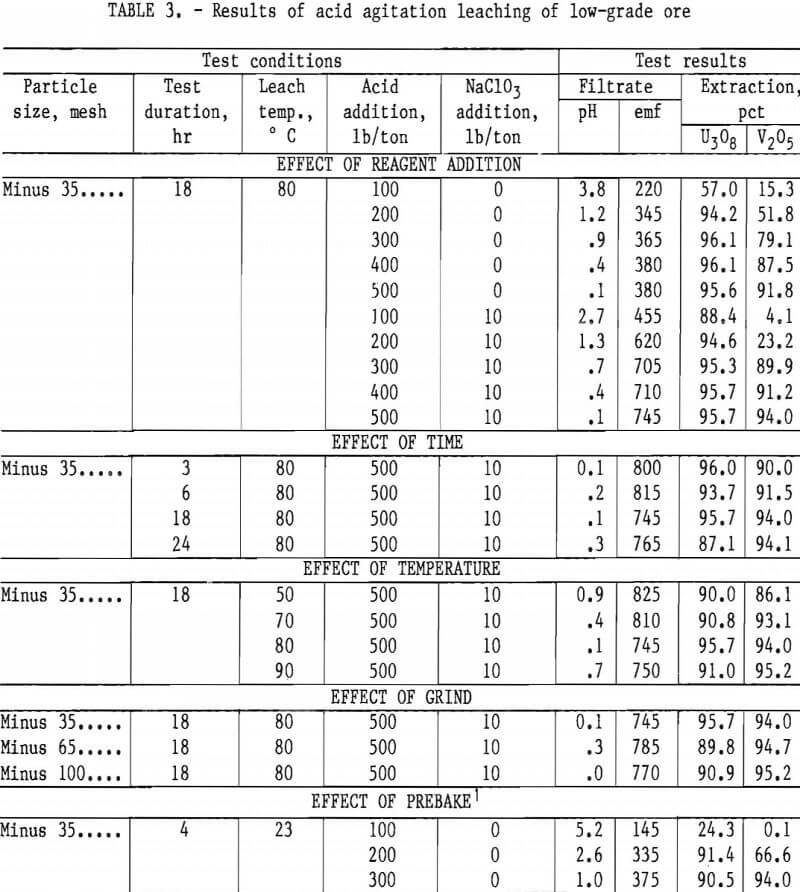
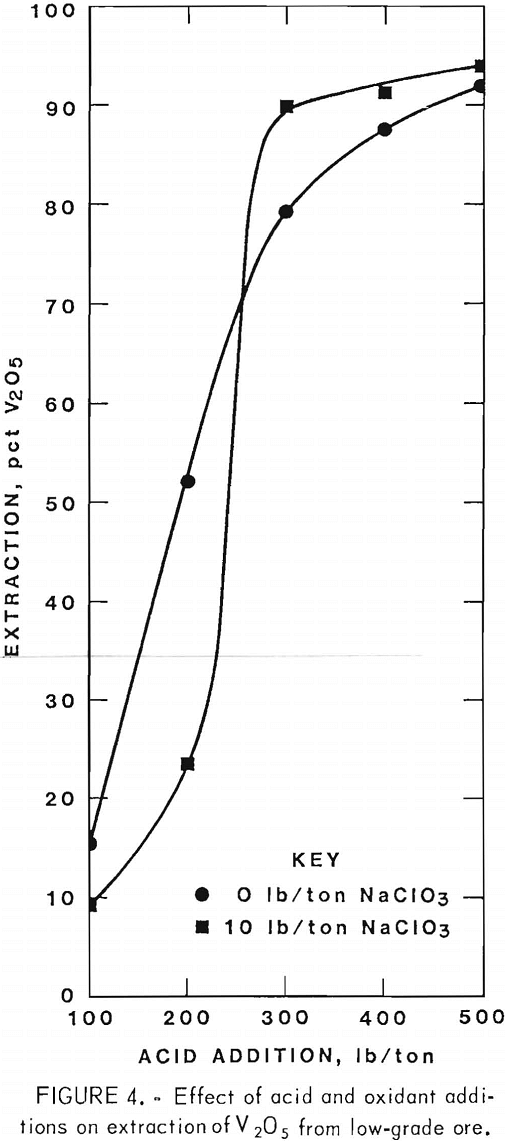
Operating conditions and leaching results for samples 1 and 2 are presented in tables 2 and 3, respectively. Figures 3 and 4 show the effects of acid and oxidant additions on the extraction of V2O5 from the two ore grades; vanadium extractions start leveling off at 300 lb/ ton acid for the mill-grade ore and at 300 lb/ton acid and 10 lb/ton NaClO3 for the low-grade ore.
Results presented in table 2 indicate the best combination of acid and oxidant with a mill-grade sample produced a 95-pct vanadium extraction. This was achieved by using 400 lb/ton H2SO4 and 10 lb/ton NaClO3. Increasing acid concentration from 400 to 500 lb/ton in-creased the vanadium extraction by less
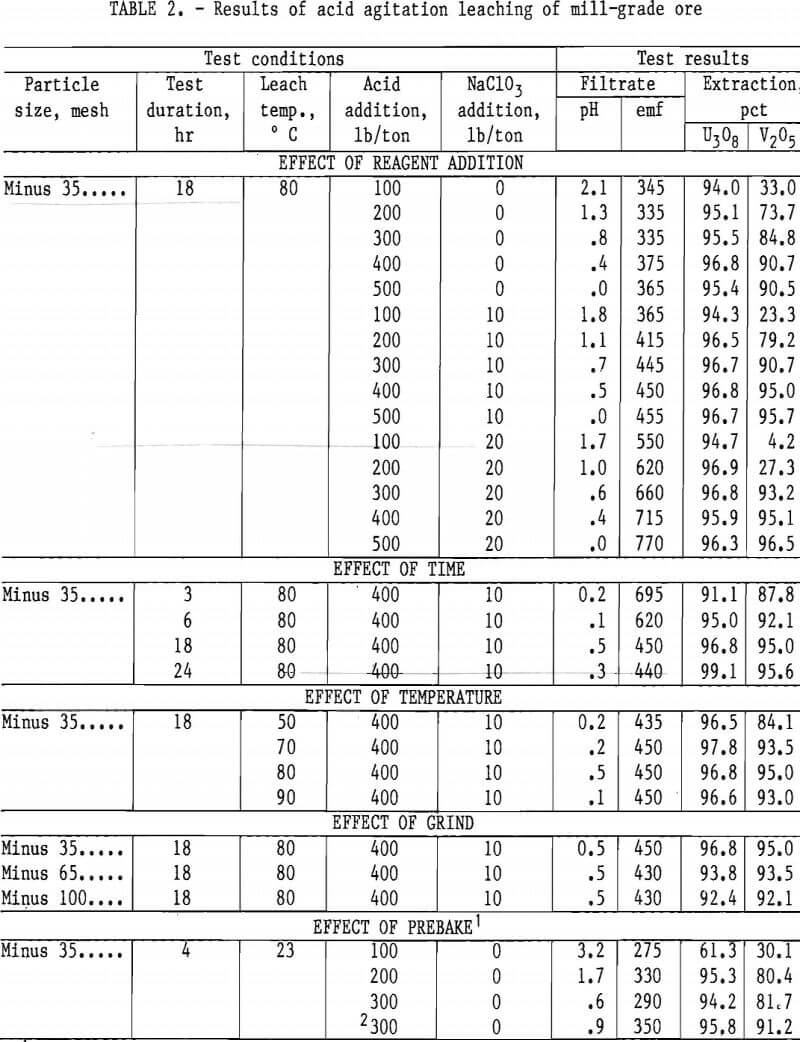
than 1 pct, and no significant advantage occurred when the NaClO3 addition was increased from 10 to 20 lb/ton. Increasing the acid concentration from 200 to 400 lb/ton produced a significant vanadium extraction increase; the uranium remained unaffected. The best operating conditions for both vanadium and uraniuextraction were an 18-hr leach time, an 80° C leach temperature, and a minus 35- mesh ore.
Prebaking of acid-pugged mill-grade ore prior to agitation leaching was used as a possible means of reducing the acid consumption. Results shown in table 2
indicate the addition of 300 lb/ton acid did not improve extraction more than the regular agitation leach process.
As with the mill-grade sample, considerable acid was required to extract vanadium from the low-grade sample; the uranium was consistently extracted regardless of the reagent concentrations. When the acid addition was increased to 500 lb/ton with 10 lb/ton NaClO3, 94-pct vanadium extraction was obtained from a low-grade ore sample, as shown in table 3. An addition of 500 lb/ton H2SO4 and 10 lb/ton NaClO3 as an oxidant was used for the agitation leach tests where time, temperature, and grind were compared. The best operating conditions again were an 18-hr leach time, and 80° C leach temperature, and a minus 35-mesh ore.
In contrast to the mill-grade ore, pre-baking of the acid-pugged low-grade ore appears to show some promise for reagent reduction when the ore is pugged with 300 lb/ton H2SO4. Although the uranium extraction was reduced to 90.5 pct, the vanadium extraction increased from 79 to 94 pct when the ore was pre-baked with no oxidant added.

Roast-Leach Tests
Roasting with or without NaCl has been used to improve leaching characteristics of some vanadium-uranium ores since the 1930’s. Roast-leach procedures are often successful in removing carbon, improving filtration, and solubilizing sodium vanadate. In recent years, environmental and economic considerations have eliminated the roasting process. As more refractory ores are used, however, it may prove advantageous in the future to reestablish this roasting method.
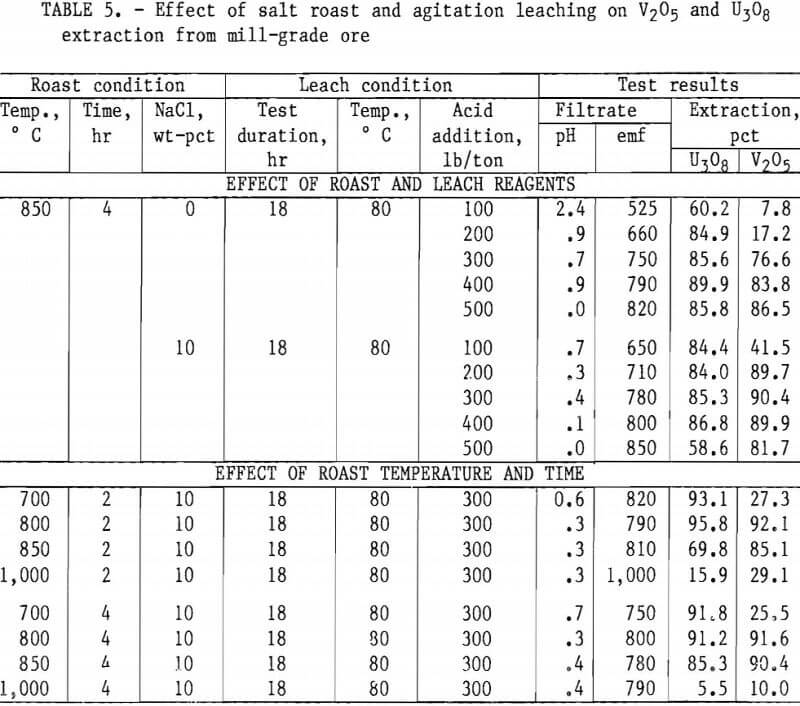
Taking into consideration the mineralogical composition of both the mill- and low-grade samples, a series of roast- leach studies was conducted to determine the effect of a roast on the extraction of vanadium and uranium. As in the previous experiments, 200 g of minus 35-mesh ore was processed. Samples were placed in ceramic boats and heated in a muffle furnace at the desired temperature for the predetermined time. By intermittent rabbling during roasting, oxidation of the sample material was achieved and fusing of the particles was prevented. At the completion of the roasting period, the calcines were air-cooled to room temperature and then leached with H2SO4. Test conditions for this leach procedure were 18-hr leach time, 80° C leach temperature, and 50-pct-solids pulp density. Tables 4, 5, and 6 present the results of this type of ore treatment for the two samples.
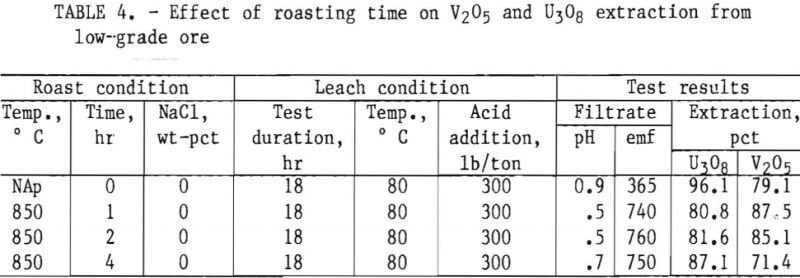
Results listed in table 4 show the effect of roasting time on vanadium and uranium dissolution from the low-grade ore. Preliminary roast tests were performed with no NaCl additions at a furnace temperature of 850° C. A comparison is made of 1-, 2-, and 4-hr roastings, along with a similar experiment with unroasted ore. Test results show V2O5 extraction for low-grade ore is improved by a short roasting period at 850° C. The longer the roasting process continued at 850° C, the poorer the vanadium ex¬traction, probably because of the formation of insoluble vanadium complexes.
Tables 5 and 6 present results of tests designed to compare the effects of reagent additions, roasting temperatures, and roasting periods on metal extractions. The results in table 5 show the best salt-roast leach extraction on mill- grade ore was obtained with a 2-hr roast at 800° C with 10 pct salt, followed by an 18-hr agitation leach at 80° C using 300 lb/ton H2SO4. At these specific test conditions, a vanadium extraction of 92 pct was attained. Table 6 shows the best salt-roast leach extraction with low-grade ore was obtained with a 4-hr roast at 800° C with 10 pct salt, followed by 18-hr agitation leaching at 80° C using 300 lb/ton H2SO4. A vanadium extraction of 38 pct was achieved under these conditions.
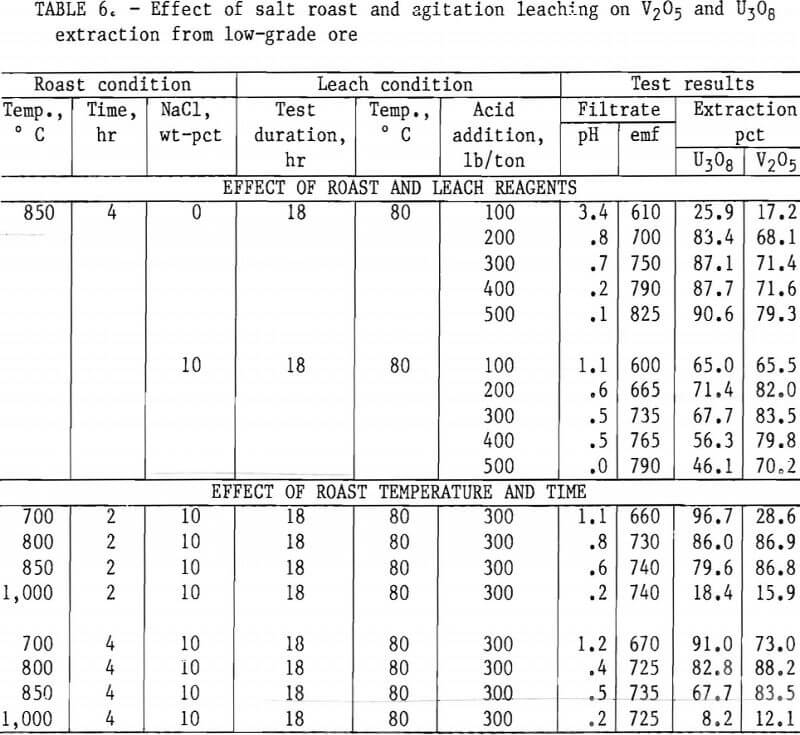
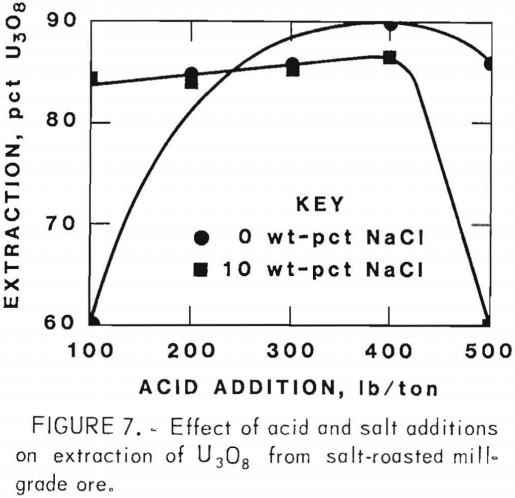
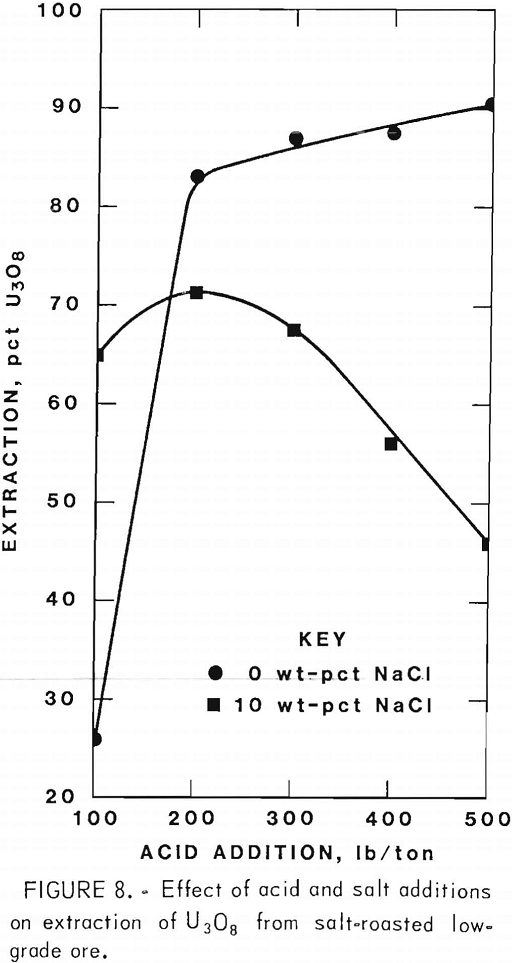
Figures 5 through 8 illustrate portions of the data presented in tables 5 and 6, which show the effect of acid and salt addition on V2O5 or U3O8 extractions. These data were gathered using results from ore that was roasted with different
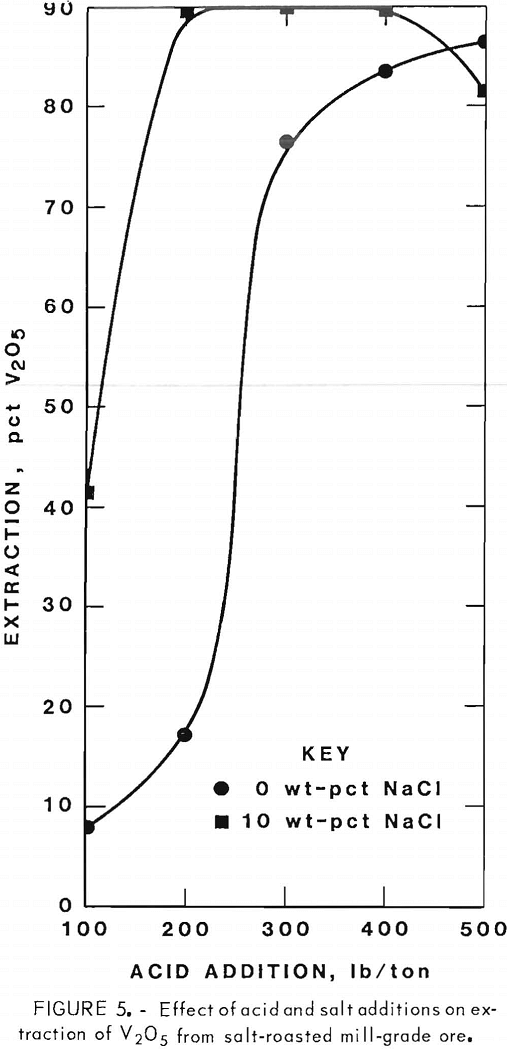
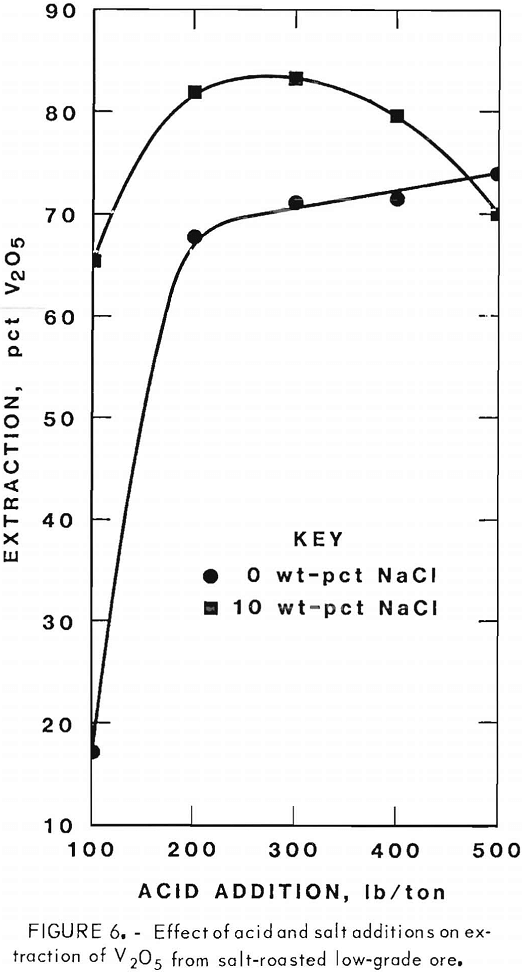
NaCl additions for 4 hr at 850° C and then leached with various acid concentrations. A comparison of figures 5 and 6 shows that leaching the two grades of vanadium ore produced similarly shaped curves but better vanadium extraction was attained from the mill-grade ore. Figures 7 and 8 compare the extraction of U3O8. A point was reached in each series where additional acid was detrimental to extraction of both vanadium and uranium when NaCl was used. The best compromise roast-leach results were produced by roasting without NaCl and then leaching at an acid concentration of 300 lb/ton H2SO4. The extraction of V2O5 was 71 pct and U3O8 was 87 pct. Although salt roasting increased vanadium extraction, it was detrimental to uranium extraction.
Autoclave Leaching
The third technique examined for processing the two ores was oxidative autoclave leaching. Because of the nature of this type of ore processing, the effects of the following operating parameters were examined: (1) acid addition, (2) leach duration, (3) temperature, and (4) pressure.
Tests were performed using a Series 4500 2-L Parr autoclave. Figure 9 illustrates equipment used in the bench autoclave leaching experiments. Fifty grams of minus 35-mesh ore sample were combined with 200 mL of lixiviant consisting of varying amounts of H2SO4 in water. This gave a standard pulp density of 20 pct solids, which was conducive to proper agitation. After leaching, the filter cake was separated from the pregnant leach liquor using a Buchner vacuum funnel. The leach solution pH and emf
were measured. Two volumes of pH 1.5 solution and one volume of water were passed through the filter cake. These wash solutions were combined with the pregnant leach liquor, and the total volume was measured prior to sampling and analysis. The cake was dried, sampled, and analyzed for V2O5 and U3O8.

Tables 7 and 8 present experimental operating conditions and extraction results for the two ore samples. The effect of leach duration was examined for each ore. Increasing the leach time from 1.0 to 5 hr increased the amount of V2O5 extracted approximately 17 pct with the mill-grade ore and approximately 3 pct with the low-grade ore.
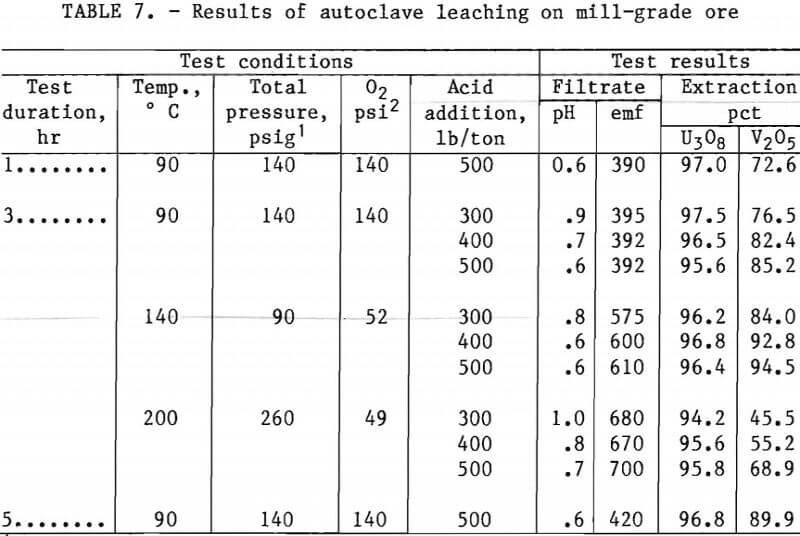
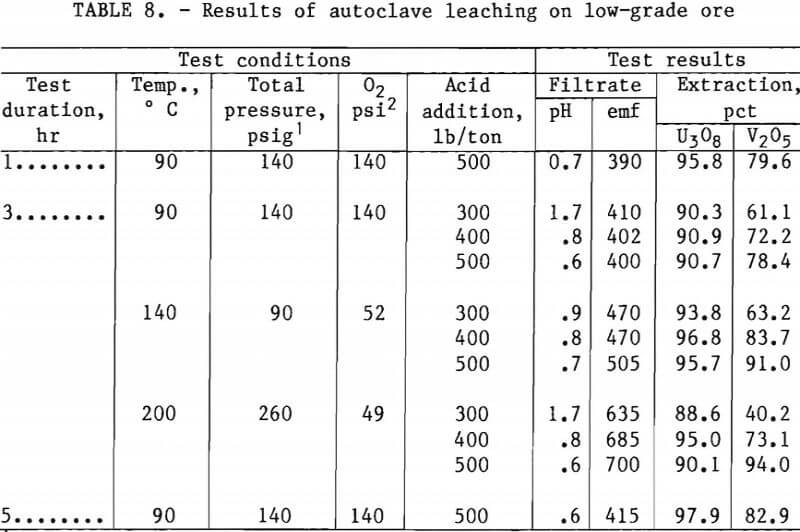
Tables 7 and 8 also show results obtained when variations were made in acid addition, temperature, and oxygen over-pressure. A V2O5 extraction of 93 pct was produced by autoclave leaching the mill-grade ore for 3 hr with an acid concentration of 400 lb/ton at 140° C with about 50 psi oxygen overpressure. When the acid concentration was increased to 500 lb/ton at the same operating conditions, the V2O5 extraction increased to 95 pct. The autoclave leaching of the low-grade ore required an operating temperature of 200° C to extract 94 pct of the vanadium from the ore. This was accomplished with 500 lb/ton H2SO4 and about 50 psi of oxygen overpressure.
Summary and Conclusions
Although leaching requirements for the low- and mill-grade ores were generally similar, the following conclusions are possible.
- Acid concentrations of 100 to 200 lb/ton were sufficient to extract over 90 pct of the uranium from the two ores treated by agitation leaching; 300 to 500 lb/ton was required to extract over 90 pct of the vanadium.
- The best combination of conditions for the mill-grade ore agitation leach included additions of 400 lb/ton H2SO4 and 10 lb/ton NaClO3 in an 18-hr duration leach at 80° C. Resulting vanadium and uranium extractions were 95 pct and 97 pct, respectively.
- Agitation leaching of the low-grade ore using similar conditions resulted in extraction of 91 pct of the vanadium and 96 pct of the uranium. Increasing the acid to 500 lb/ton increased the vanadium extraction to 94 pct with no effect on the uranium.
- Use of a prebake pugging technique resulted in a lower acid requirement. A 94 pct vanadium and 91 pct uranium extraction was achieved using 300 lb/ton acid addition with no NaClO3 addition for the low-grade ore.
- The best salt roast-leach results on the mill-grade ore were obtained with a 2-hr roast at 800° C with 10 pct salt followed by an 18-hr leach at 80° C and 300 lb/ton H2SO4. The vanadium extraction was 92 pct under these conditions.
- The best salt roast-leach results on the low-grade ore were obtained with a 4-hr roast at 800° C with 10 pct salt followed by an 18-hr leach at 80° C and 300 lb/ton H2SO4. The vanadium extraction was 88 pct under these conditions.
- Salt roasting improved vanadium extraction with both ores, but was detrimental to uranium extraction.
- Autoclave leaching of the mill- grade ore extracted 93 pet of the vanadium in 3 hr with an acid addition of 400 lb/ton. Increasing the acid input to 500 lb/ton increased the extraction to 95 pct. These tests were run at 140° C with about 50 psi oxygen overpressure.
- To attain 94-pct vanadium extraction on the low-grade ore with a similar autoclave test using an acid addition of 500 lb/ton required increasing the temperature to 200° C.
- The recovery of vanadium and uranium from low-grade carnotite ores appears to be feasible. The choice of a processing method, however, would depend in a variety of economic and environmental factors.
