Bleaching clays have been used extensively in the oil-refining industries for a number of years. Their use in water purification is relatively recent and less extensive. They are frequently classified as (1) those naturally active and (2) those active after artificial activation with acid. Fuller’s earths fall within the general group of bleaching clays and gradually the commercial term “fuller’s earth” has been applied to natural clays originating, for the most part, in the southeastern section of the United States. Bentonitic clays are usually classed as activable and are prevalent in the southwestern section of the United States.
Bleaching clays vary, considerably in chemical and physical properties throughout the country, or even in different parts of one deposit (Table 1).
The fuller’s earth type of clay is characterized by a lack of plasticity, relatively adsorbent, foliated structure, and does not swell to form a permanent sol. The bentonitic clays, on the other hand, are characterized, in general, by a waxy appearance and the quality of slaking in water. They have less valuable adsorbent properties but swell to a heavy gel and in more dilute suspensions form sols that may remain in suspension indefinitely.
The best grades of bleaching clays are light gray to brown in color in the raw, wet state, and nearly white after drying. Activable clays, as a rule, are heavier and denser than fuller’s earth clays. Although all clays possess many similar characteristics, slight differences in composition greatly affect their specific function and value in water treatment. Fuller’s earth is known to the oil-refining trade as “bleaching clay” and to the water works superintendent as “adsorbent or water-conditioning clay.” Many clays used in water purification are surface mined, dried, ground to a fine size, and packaged for shipment in either burlap or paper bags each containing 100 pounds.
Advantages of Bleaching Clay
Bleaching clay is a comparatively recent addition to water-purification materials. It bids well to become a most useful substance in proper coagulation and control of taste and odor. It is inexpensive, easy to apply (it can be fed through the conventional type of dry-feed machine usually found at the average water-purification plant, Fig. 1). It is nonchemical in nature and capable of forming a strong and adsorbent floc in many waters having a turbidity of 100 p.p.m. or less.
Clay is used in water purification primarily as a coagulant or floc-building agent; it adds toughness and weight to the floc particles, which accelerates coagulation, enmeshing colloidal suspended material and some bacteria into jell-like aggregates that settle rapidly, sweeping down suspended materials (hydrous aluminum oxide) into the sludge areas of the sedimentation unit, thus permitting the resultant relatively clear supernatant water to flow on to the sand and gravel filters for final clarification.
In many instances this superior floe formation has materially reduced at the outset of the purification process substances that produce taste and odor, thus substantially reducing the cost of water purification and the possibility of subsequent intensification as the process continues.
Bentonite may be used advantageously as a coagulant in water having a relatively high hardness (calcium-magnesium) content. It is especially effective as a coagulant in the presence of magnesium sulphate. Each clay performs a definite function according to the quality of material used and character of the water treated. The ability of clays, especially bentonites, to swell in water is said to be due to the expanding of its latticelike structures by capillary pressure, pushing apart the stable film around each particle. This ability of clay to swell in water may increase its volume more than 100 per cent.
Careful examination of many silt-bearing raw waters will disclose that the individual particle of silt or clay in suspension is practically covered with a slimy coating of organic matter. Such a condition frequently tends to resist further adsorption and coagulation. This may be brought about in part by the character and colloidal nature of the slime-coated suspended material. The addition of adsorbent clay to many water-purification processes constitutes a third colloidal dispersion agent, which frequently brings about a coalescing effect between the original suspended particle with solutions of alum and results in the production of a crystal-clear purified water.
The application of adsorbent clay in the process of water purification may accomplish one or more of four essential functions in treatment plants: (1) Rapid settling of suspended material is frequently attained in conjunction with the regular coagulant (widens hydrogen-ion concentration-coagulation range); (2) certain substances contributing to taste and odor (especially those due to oily waste, etc.) may be reduced or entirely eliminated; (3) coloring matter imparted to the water from vegetable sources is appreciably reduced, especially when used with alum coagulation; (4) clay has been found to exert certain zeolitic effects on many hard waters and thus reduces the cost of water softening.
Dr. Edward M. Slocum, Research Chemist of the General Reduction Co., Macon, Ga., was an early advocate of the use of clay in water purification. Dr. Slocum has had international experience with clay in the oil and perfume industries. His continued interest and suggestions have been largely responsible for the present use of clay in water purification.
One of the first successful applications of clay in water purification was made by R. I. Dodd, Chemist with the Chester Water Service Co., Chester, Pa. He found that the application of clay at the intake well prior to the application of alum in the mixing chamber successfully reduced troublesome oily trade-waste odors in the water. Putrefaction of organic matter in the sedimentation basin was noticeably reduced. Breaking up of the floc in cold weather was also overcome by the formation of a stronger and more adsorbent precipitate. Approximately 43 lb. of clay, costing-about $0.35 per million gallons, was used at Chester.
George D. Norcom, Sanitary Engineer of the Federal Water Service Corporation, reports, after considerable investigation, that the use of clay in the treatment of Delaware River water effectively produced a more palatable water at a nominal cost.
Carl R. Alexander, Superintendent of Water Works at Rome, Ga., reports some very interesting data on the use of clay at an industrial rayon-manufacturing plant. This plant derives its supply from the Oostanaula River. The turbidity of the river water varies from 20 to 2000 p.p.m. It is essential that filtered water used in the manufacture of rayon shall not exceed 0.1 p.p.m. turbidity.
Many different combinations of coagulants were used in an endeavor to attain the desired results. Before clay was used in coagulation, it was practically impossible to obtain a filter effluent of less than 0.4 to 0.15 p.p.m. The application of 40 to 60 lb. of clay, with alum, not only produced a larger floc, which settled more quickly/but also reduced the turbidity of the filtered water to 0.05 p.p.m. This combination of clay and alum produced a water of superior quality and at the same time reduced the cost of chemical application.
John R. Baylis, Physical Chemist, Chicago, Ill., has tested a number of different types of clay in the laboratory of the Chicago Experimental Water Treatment Plant. He concludes that clays are of assistance in the formation of a heavy and rapidly settling floe on Lake Michigan water. However, his results in control of taste and odor did not indicate appreciable reduction.
Odell W. Gray, Chemist, Water Softening Plant, Thomasville, Ga., reports that when clay was used as the principal coagulant in water softening the hardness of the finished water was substantially reduced. The increased efficiency of coagulation produced a superior finished water both chemically and bacteriologically. Filter runs were increased from 40 to 150 hr. between washings. The application of clay was responsible for a 10 per cent saving in chemicals.
H. L. Olin, Professor of Chemical Engineering, University of Iowa, and collaborators J. V. Gauler and H. W. Peterson, have published two outstanding treatises on the use of bentonite clays in water purification. Professor Olin has spared neither time nor detail in the compilation and execution of this invaluable research project. The report indicates that certain alkali bentonitic clays (Utah and Wyoming) readily coagulate in the presence of dissolved mineral matter to form a heavy, gelatinous floc, which filters rapidly, giving a sparkling, clear water. These clays are slightly zeolitic.
They have little effect on reducing vegetable coloring matter in water. Utah bentonite clays swell about eleven times their original size in water. The use of this clay as a coagulant reduced the carbonate hardness of the Iowa River water from 250 to about 200 p.p.m.; 40 p.p.m. of bentonite reduced the total hardness of the river water to 100 p.p.m. Professor Olin states that the use of sodium bentonite as a coagulant is relatively independent of the pH value of the water treated. Lime softening of a water containing carbonate hardness presents a particularly favorable situation for the use of bentonite as a coagulant in clarification.
Use of Clay at Atlanta
Increased rate of consumption at Atlanta, together with a pronounced oily pollution in the Chattahoochee River water, materially taxed the normal operation of the purification plants in producing a satisfactory finished water. Two conditions were most evident: (1) Insufficient time for mixing and settling resulted in light floe formation; (2) taste and odor complaints caused by increased rate of plant operation and complexity of treatment.
Many experiments were made in an endeavor to attain greater plant efficiency by rearrangement and combination of existing material and equipment. Activated carbon was effective in materially reducing unpleasant taste and odor, but did not aid appreciably in the formation of a tougher and more rapidly settling floc.
Innumerable jar tests were conducted on the river water during this period, using both fuller’s earth and bentonite. The most successful of these tests were subjected to plant-scale trials. Natural fuller’s earth was used for this purpose because of its availability and favorable cost. (See attached specifications, Table 2.)
Three different points of application were studied on plant-scale tests to determine the most effective combination: one had alum added about five minutes before clay, another had alum and clay added simultaneously and the third had alum added about five minutes after clay. The application, of alum and clay at a common point proved most conducive to a quick-settling floc. This procedure has been used at Atlanta for more than four years when raw water turbidities are less than 100 p.p.m.
The amount of clay used in these early tests varied from 2 to 250 lb. per million gallons with an average of about 20 lb. per million gallons. The amount of alum used averaged 65 lb. per million gallons. During this time the quantity of carbon applied in the last section of the sedimentation basin was reduced from 15 to 8 lb. per million gallons. Daily threshold odor tests control the application of clay and carbon.

The City of Atlanta reserves the right to accept or reject any or all bids and to award the contract to the best interest of the City, all technicalities waived.
The requirements of the City of Atlanta for the year 1937 are a minimum of 40 tons and a maximum of 100 tons, for delivery during the year 1937.
Deliveries to be made f.o.b. Atlanta Water Works, Hemphill Pumping Station, Southern Railway, Atlanta, Georgia, in such quantities and at such times as the City of Atlanta, through its proper officials, may direct.
Note: A copy of the above specifications was sent to each firm submitting a bid on Atlanta’s Clay requirements for 1937. The contract was awarded to the firm submitting the highest quality product in the opinion of the City.
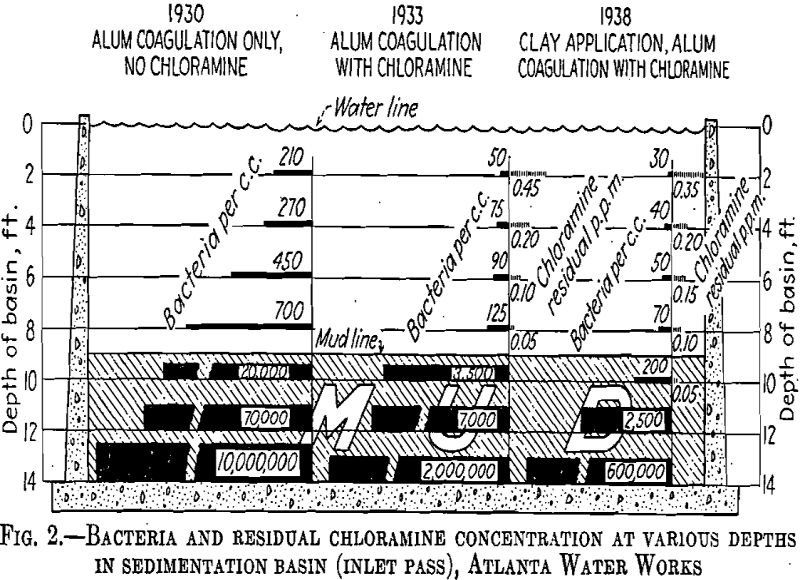
Observations at Atlanta indicated that clay has little or no action on chlorine applied to the raw water. On the contrary, chlorine appears to be held in the surface of the coagulated masses. This maintains the deposited sludge in the sedimentation basin in a fresh condition. In 1930 (Fig. 2) at the bottom of one of our sedimentation basins, the bacterial count was 10 million; in 1933 chlorine sterilization of the raw water reduced this count to 2 million and in 1938 the application of clay reduced the count to 600,000. We believe that this improved condition was brought about by the presence of chlorine-laden clay in the lower strata of the basin. It is the custom at Atlanta to measure the depth of
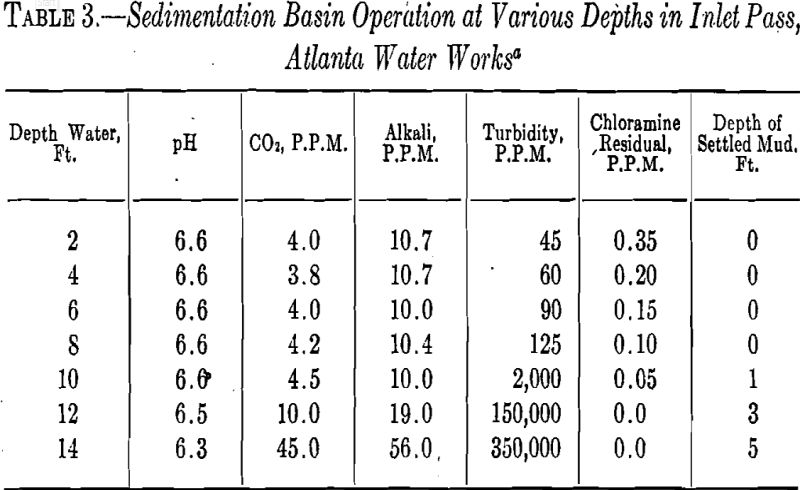
sludge in the sedimentation basins each month. A typical cross section of these measurements will be found in Table 3.
About a year ago an auxiliary mixing chamber was built in order that an additional volume of coagulated water might be obtained to supplement that used through the regular process. This basin is used intermittently. It has a capacity for 4 to 15 million gallons daily. The supply is derived from a second river-water reservoir in which there is a minimum of circulation, permitting a luxuriant growth of microorganisms.
The turbidity of this water seldom exceeds 50 p.p.m. It is colored and susceptible to very unpleasant odors. The average period of detention of this chamber is only 15 min., or 1/3 that of„the regular unit. This necessitates larger amounts of coagulant.
Last fall alum was used in this mixing chamber without clay. The filter runs were reduced from 120 to 71 hr. and the cost of wash water increased appreciably. However, when 40 lb. of clay per million gallons was added, with alum coagulation, the filter runs were restored to normal and the cost of wash water reduced 40 per cent (Table 4).

The regular use of about 20 lb. of clay at a cost of 10¢ per million gallons, with alum application, has increased the efficiency of water treatment at Atlanta from 10 to 30 per cent.
Conclusion
Fuller’s earth or adsorbent clays materially improve alum coagulation on many waters having a turbidity of about 100 p.p.m. or less. A heavy floc is formed, which settles rapidly and produces good coagulation, and the water filters out crystal clear.
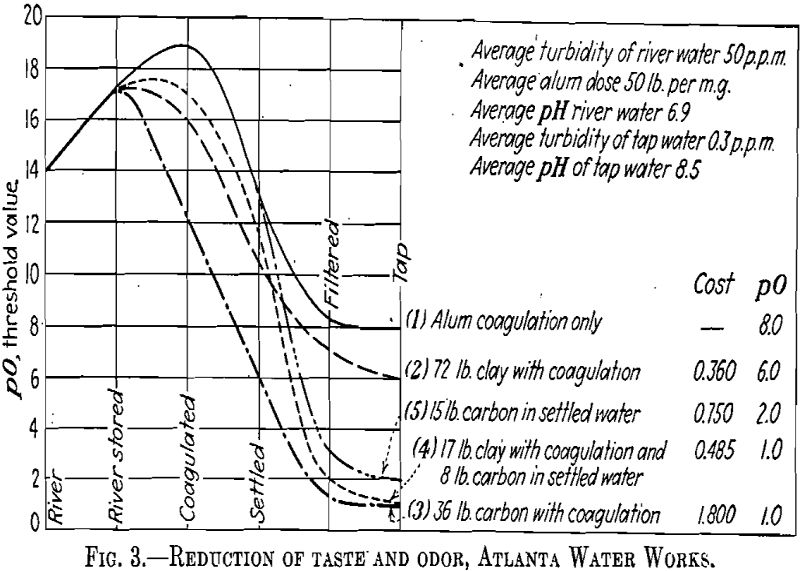
Clay is inert toward the chlorine it adsorbs. When deposited in the sludge area of many sedimentation basins it has a tendency to reduce putrescence, odors, and even coliform bacteria (Fig. 3).
When clay forms a tougher and more adsorbent, floc with alum in colored waters, there is a tendency to remove the color almost, proportional to the amount of clay used.
Frequently, objectionable materials are removed from the water before they interfere with subsequent treatment in the purification process, when clay is used with the coagulant. This insures more effective and economical use of adsorbing and sterilizing materials.
Bentonitic clays have proved effective as coagulants in a number of water-softening plants, especially when the water contains magnesium sulphate.
Clay with zeolitic properties has been known to reduce the total hardness of a relatively hard water when used instead of other coagulants.
Most water-conditioning clays are inexpensive and can be purchased for ½ to 1½¢ per pound. Large deposits of fuller’s earth and bentonitic clays, relatively undeveloped at the present time, exist in middle and southwest Georgia.
Our experience at Atlanta indicates that when clay is applied in the raw water and activated carbon in the settled water to the filters, they become complementary. Thus, their combined effectiveness to improve coagulation and reduce taste and odor surpasses that of either when used separately (Fig. 3).
There are said to be some ten thousand water-treatment plants in the United States, supplying about five billion gallons of water daily to approximately sixty million people.
It is exceedingly difficult to hazard an estimate as to the quantity of clay that might be used by these plants. The average operator is not familiar with the use of clay in water purification. If clay is to come into general use in this field, the responsibility of merchandising will be left largely to the manufacturer.
Various sources of information have indicated that about 300 tons of activated carbon are consumed daily in removing unpleasant tastes and odors from water. If clay could be substituted for, or used as an adjunct to, carbon it would require from 10 to 50 lb. for each pound of carbon used.
It is not the purpose of this paper to set up fuller’s earth (adsorbent clay) or bentonite clay as a panacea for all difficulties of coagulation, taste and odor, but rather to bring out facts and procedures that, may be of benefit in special cases.
Acknowledgments
The author wishes to express his appreciation to Mr. W. Z. Smith, General Manager of the Atlanta Water Department, for his suggestions and aid in conducting clay tests at Atlanta; to the Water Purification Plant employees, and to all others who have generously contributed data for this discussion.

Contact us here
