The presence of radioactivity in uranium mill wastes has resulted in somewhat unique waste disposal methods. In addition to the common problems of disposing of large quantities of solid wastes, neutralizing acids, minimizing dissolved heavy metals, and clarifying all liquid effluents, the uranium mill operator must sample and analyze liquid effluents for micro-micro quantities of radionuclides such as, radium-226, thorium-230 and lead-210. Special disposal methods or decontamination procedures are required to meet the stringent limitations established by the U. S. Atomic Energy Commission on the concentration of radionuclides in liquid effluents. Routine monitoring of the receiving stream is also normally required.
Description of Liquid Wastes
Uranium extraction techniques generally involve either acid or carbonate leaching of the ores after preparation by crushing, grinding and, in some cases, roasting. Soluble uranium is recovered by ion exchange, solvent extraction, or in the case of certain alkaline leach processes by caustic precipitation. The uranium barren solutions from these uranium recovery steps make up the liquid wastes containing small amounts of radioactivity and varying amounts of dissolved solids.
The ores from which uranium is recovered in western United States contain in the order of 0.20% to 0.25% uranium oxide (U3O8) , and the radioactive materials present are the normally occurring U-238 and U-235 isotopes and their decay products. The low natural ratio of U-235 to U-238 (1 to 139) automatically reduces the potential significance of the U-235 decay products.
Of the total radioactivity in uranium ore, only about 170, and in some cases much less, will leave the mill as dissolved alpha and beta activity in the liquid effluent that enters the tailing pond. The total uranium concentration in the liquid effluent is maintained below the level of 2 x 10 -5 µc/ml required by the Code of Federal Regulations. Most of the radium in the ore is insoluble and remains in the impounded tailing solids. A small portion of the radium, in the order of 1% or less, is dissolved.
Although the radionuclides Ra-223, Th-227, Ac-227, Pb-210, and Po-210 may also be present in liquid effluents, only a limited amount of work has been done to determine the quantity of other uranium-chain decay products because the Ra-226 level has been the most restrictive and consequently the most significant isotope of concern.
Description of Solid Wastes
About 14% of the total radioactivity contained in the ore fed to mills is recovered in the uranium concentrate. The short-lived radionuclides, Th-234, Pa-234, and Th-231, are subsequently lost by decay. Approximately 70% of the activity in the ore remains undissolved in the solid mill tailings, which are impounded in tailing dams.
All mills discharge tailing material containing the radioactive solids and in many mills, the liquid effluent to the tailing pond. The areas of the ponds vary from a few acres to several hundred acres. The solid tailings are commonly used to build a dam, and the liquids are retained in the pond for a sufficient time to allow settling of the residual solids. Solid tailings are sampled for mill control purposes, but routine radiochemical determinations have not been necessary because the tailings are impounded at the mill site.
Disposal of Liquid Wastes
Some uranium mills located on stream or river drainage areas have little choice but to release liquid to adjacent rivers and streams. This is the case at about one-third of the existing uranium mills. Another third of the mills, although on river drainage areas, do not discharge effluent directly to the stream. Where land area permits, the tailing pond at these mills, as well as the ponds at another third of the mills that are not located on a river or stream, are of sufficient size that the liquids evaporate or seep into the ground. In some cases the mills recycle the pond overflow back to the process either for reasons of uranium recovery, conservation of water, reduction of effluent volumes, or combinations thereof. All mills that discharge an overflow to a water¬shed attempt to discharge clear effluent clarified in a series of settling ponds. The Anaconda Company in Grants, New Mexico, has used a deep-well- injection method for liquid-effluent disposal.
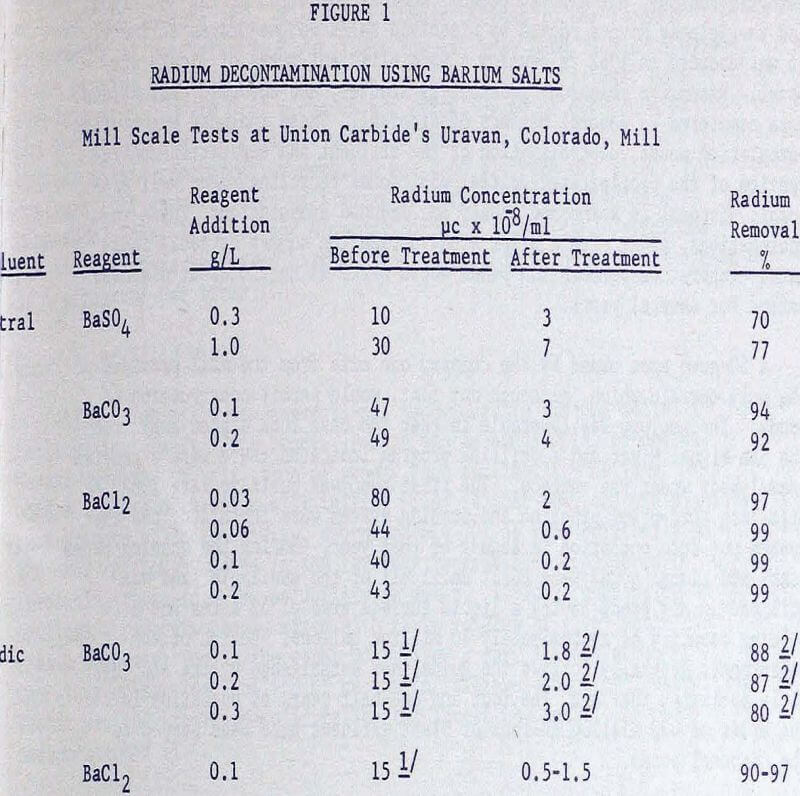
The limit on the concentration of radium in an effluent released to an unrestricted area is 3 x 10 -8 µc/ml. This level is generally attainable with the proper combination of reagent, concentration, and effluent. It was found that in most instances BaCl2 was more efficient than BaCO3, particularly in the neutral effluent, and BaCO3, in turn, was more efficient than BaSO4. Over several years of operation it was noted that the decontaminated effluent will vary in radium concentration with no obvious explanation, although it is speculated that the cause may be differences in the effluent make-up, temperature, and sudden variations in flow-rate. Efficiency of decontamination appears not to be a function of radium concentration but rather is dependent upon the make-up of the effluent.
Disposal of Solid Wastes
All uranium mills use conventional techniques to impound solid tailings in large tailing ponds. Design of the ponds will, of course, depend upon the terrain, type of ground, and availability of land. The unique feature of uranium mill tailing piles is that they contain significant amounts of radioactivity, approximately 70% of that originally in the ore. Although radium in the tailings is generally considered to be insoluble, there is a low solubility in water. If large quantities of tailings were continuously mixed with large volumes of river water the radium concentration in the water would show a very slight increase. One study on the leachability of radium in tailings showed the controlling factor was the liquid-to-solid ratio; the larger the ratio, the more radium leached.
The question has been raised as to whether uranium mill tailings, which may have reached the Colorado River or its tributaries during the earlier operations of uranium and vanadium mills or which may have been wind-blown or accidentally released in more recent years, have had an effect on the soluble radium in the river or on the radium in the sediments. This does not, however, appear to be the case.

