In Situ leaching for the recovery of uranium from low grade sandstone deposits is one of the newest technological advances in the mineral industry. It is rapidly developing into a commercially feasible mining system which has economic, environmental, and social advantages over conventional mining systems. Because of the current uranium shortage, development of In Situ leaching into a sophisticated system has gained new impetus. In Situ leaching will become an important mining technique in the future, which will greatly help to supply uranium for our nation’s energy needs.
Environmental Overview
From the environmental standpoint, solution mining of uranium shows a negligible effect on such factors as surface disturbance, interference with natural groundwater quality and distribution, and aerial discharge of radionuclides. In the surface disturbance category, only one to two pounds of tailings per pound of uranium result in acid leaching and virtually none in the carbonate leaching system, which compares very favorably to the half ton of waste produced per pound of Uranium produced from conventional systems. Additionally, the only other surface disturbance is that of clearing the area of brush and trees and grading roads to the area.
State-Of-The-Art
Solution mining requires some specific geological conditions to allow recovery of uranium. These conditions are the following:
- The ore body generally must be a horizontal bed underlain by impermeable strata for containment of solution.
- The ore body must be located below the static water table so the solutions may flow.
- The mineralogy of the deposit must be amenable to the process.
- Even though the uranium content requirement for profitable operation is much lower than in conventional operations, it must be ascertained whether the mineral content is enough for the geological conditions of the site to repay all exploration, development, and extraction costs.
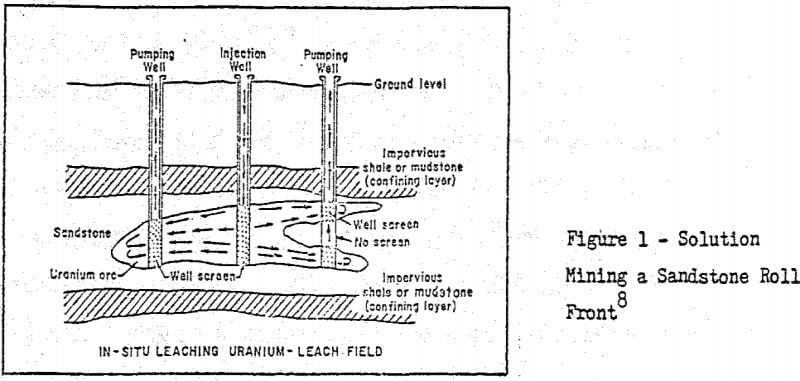
Below is a figure showing a typical sandstone roll front which pictorially describes the solution mining method. Most of the leachable uranium deposits are of this type. Of all the western uraniferous deposits, it appears quite possible that 30 to 50 percent are amenable to recovery by in situ leaching technology.
Leaching Solutions
The two principle lixiviants (leachants) used in leaching uranium are acids and alkaline carbonates. The acids used are mainly nitric and sulfuric acids. Their advantages are high yields and fairly efficient recoveries. However, because large amounts of impurities are also solubillzed and the acid cannot be regenerated, which results in high reagent consumption, carbonate lixiviants have found, widespread use in the uranium solution mining industry.
The most popular carbonate used is ammonium bicarbonate. It is non-corrosive, shows high selectivity in solubilizing uranium, requires simple procedures for recovering high grade uranium, and the reagent consumption can be kept low because the reagent may be regenerated. In a conglomerate ore, carbonate lixiviants would not be effective in recovering uranium because it could not dissolve other minerals encasing particles of uranium. However, in sandstone-type ores, the uranium is usually a precipitate on grain surfaces, which are exposed to solution flow. This makes ammonium bicarbonate an ideal lixiviant in uranium solution mining.
Problems with In Situ Leaching
Despite the attractive picture displayed so far, uranium solution mining does have its problems. Many companies experience decreased injectivity of their injection wells as leaching progresses. This is thought to be the result of precipitation of minerals or the swelling of clays. Companies have flushed their wells with solutions of strong acids which have helped increase production for a time, but the wells still seem to lose most of their permeability over time. Reverse pumping does help out in some cases also, but is not totally effective. This is a major problem of concern to many companies and research is being conducted to discover the problem (or problems) and to find solutions.
A second area of concern is the usage of reagents by minerals other than uranium. Indications of leaching studies seem to show that feldspars may be a consumer of carbonates which, depending upon the minerology of the feldspar, can give different leaching rates and reagent consumption. Calcite and pyrite dissolution are always problems which need to be controlled. Further, other minerals may be interferring in the process which are not yet suspected and need to be identified in order to get optimal results in leachant usage.
Computer Simulation
In an attempt to optimize leaching technology, computer simulation of the hydrology has been done and the mass transport and chemistry will be in the future. The thrust of simulation is to help determine the optimal well spacing, input and out put flow rates, and other parameters characteristic to the individual orebodies. This information is computed using the inputs of aquifer, well, and inherent flow characteristics.
The Bureau of Mines’ Twin Cities Mining Research Center has developed a five-spot simulation program (5-SISL program). The model was developed directly from solutions to the Hantush and Jacob hydrology equations in various closed forms. These equations describe fluid flow in a variety of aquifer types. The 5-SISL program has some options to handle the many different aquifer characteristics. The output is in the form of numerical and graphic representation of streamline isovelocity, isotime, and isopressure gradients. This helps the uranium producer to see what would occur and to determine the best well field design for a pilot 5-spot operation.

