Table of Contents
Mechanism of Uranium Leaching: Uranium leaching with sulphuric acid is a complex process governed by chemical dissolution, and the oxidation of uranium. It is controlled by surface etching, diffusion, and mass transfer within the ore. The physical parameters are in turn controlled by the physical properties of ore, and the ore size.
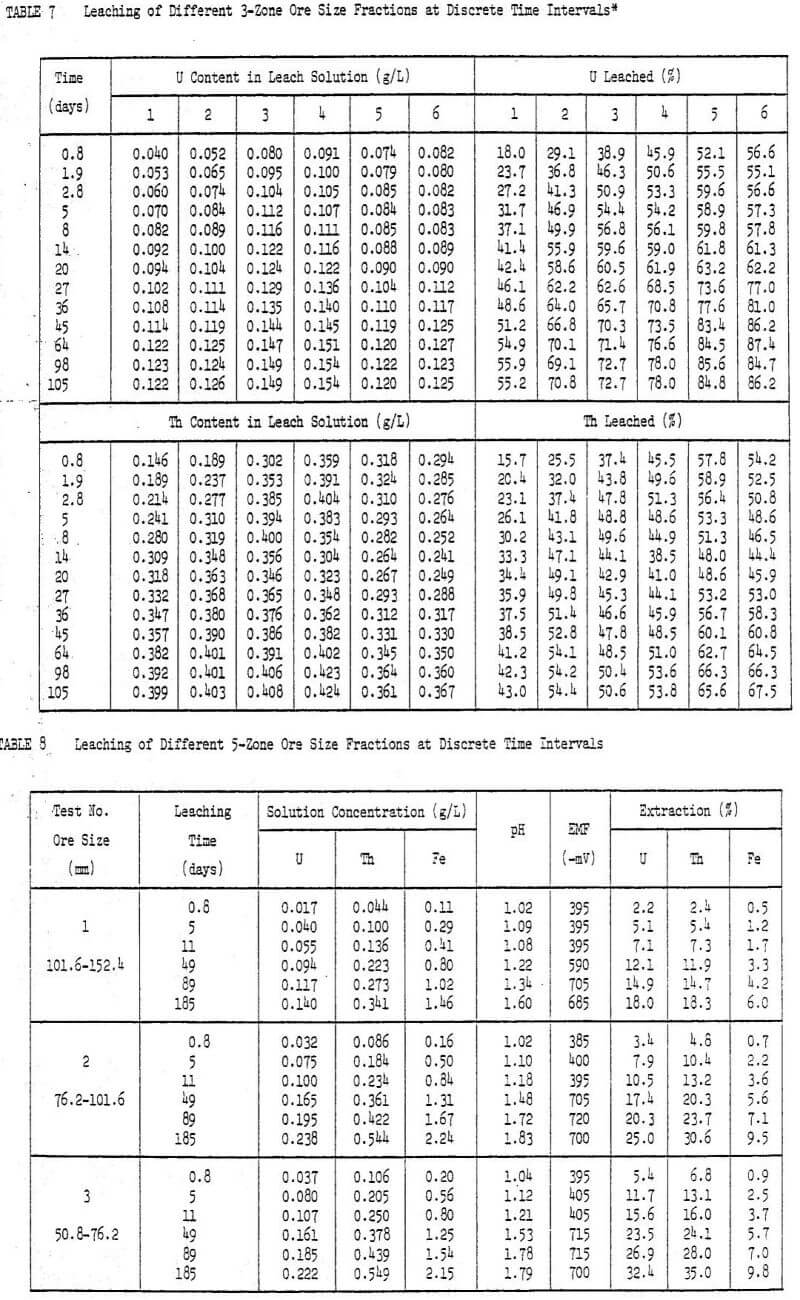
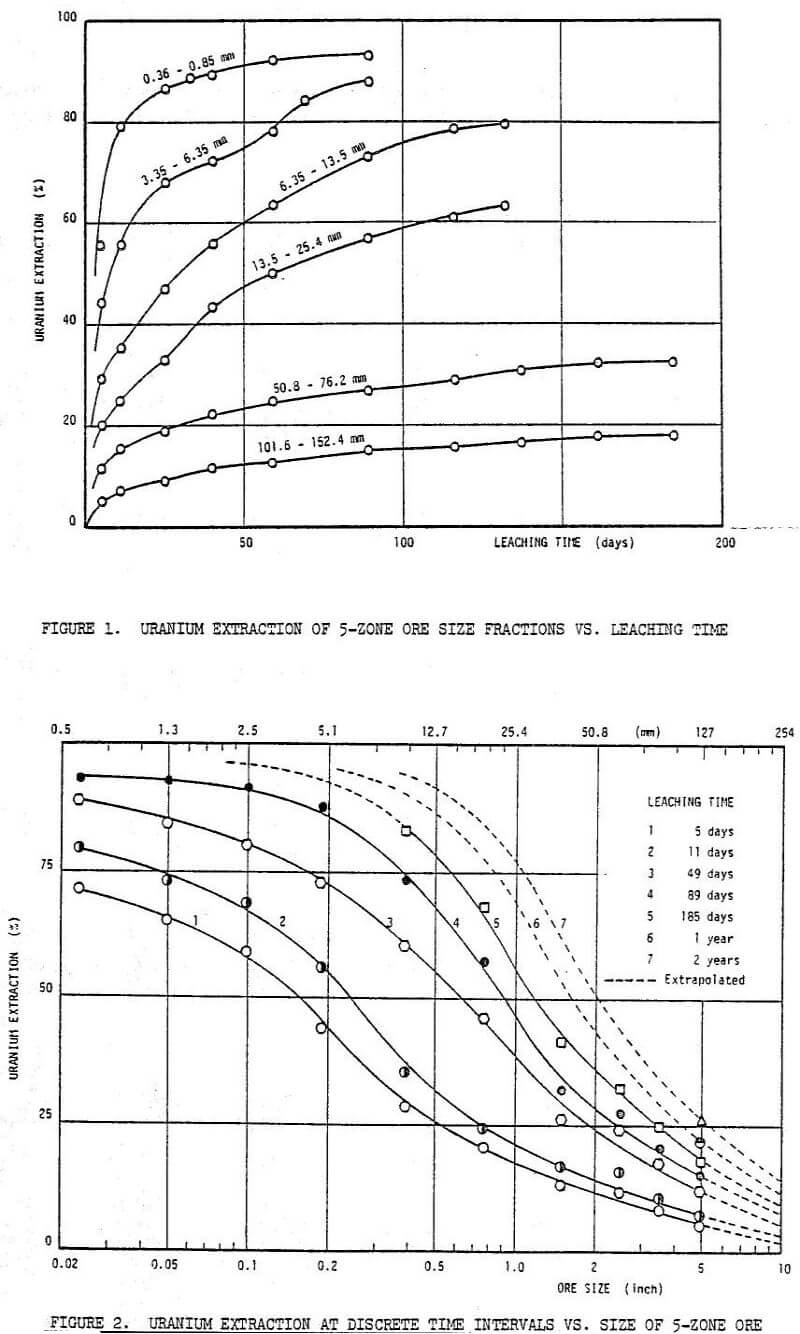
The leaching process may be conceptually divided into four basic phases. During the first phase of leaching (first 5 to 10 days), the accessible, soluble uranium minerals are solubilized by dilute sulphuric acid. This phase is generally controlled by the chemical reactions involving the ore minerals and sulphuric acid. The bacteria are not yet active and under this weak oxidizing condition (EMF around -400 mV), 70% uranium and 73% thorium are dissolved from an ore fraction of 0.36 to 0.85 mm after only 5 days of leaching. For more details refer to Table 8.
During the second phase, from the 5th to the 30th day (time could vary according to the leaching conditions), the process is more controlled by diffusion and the leaching rate decreases. The oxidation is still weak. At the end of the second phase the bacteria start to proliferate and the system proceeds to the third phase.
Once the bacteria proliferate, more Fe(II) is oxidized to Fe(III), the pH increases, and EMF changes quickly from approximately -400 mV to -700 mV. U(IV) is oxidized to U(VI) and simultaneously solubilized and the leaching rate improves. This stage lasts approximately until the 45th day of leaching.
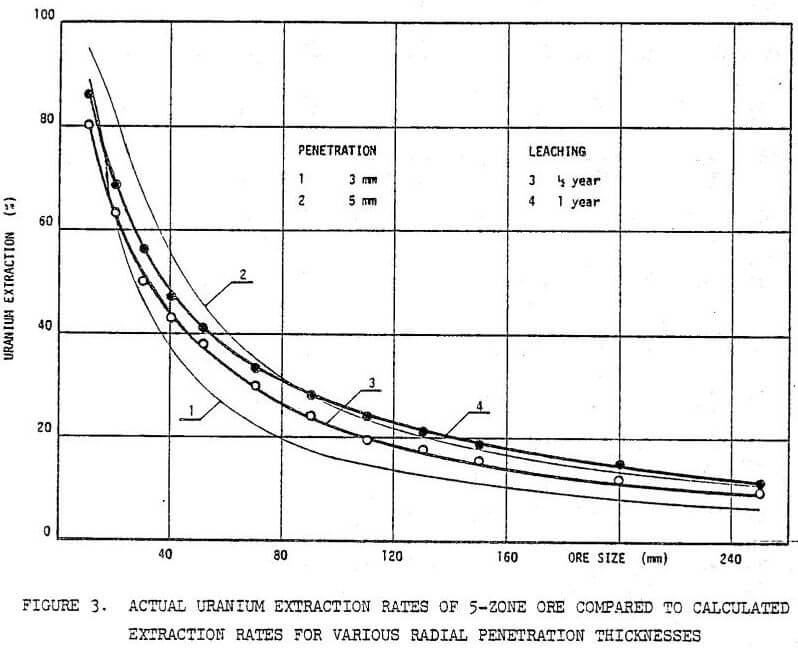
Once the accessible U(IV) is oxidized, the process is again controlled by etching and diffusion within ore. The leaching rate gradually decreases. This decrease in the extraction rate during the last stage of leaching might also be attributed to the depletion of uranium in the ore.
If the leaching is performed with a solution containing the active iron oxidizing bacteria and sufficient Fe(III) ion for the oxidation of U(IV) to U(VI), the first three phases become difficult to separate. The uranium extraction rate will be higher, initially. However, after sufficient time, the overall uranium recovery will be similar to the phase 4 case.
Mechanism of Thorium Leaching
Agnew lake ore contains about three times as much thorium as uranium. The testwork shows that thorium is extracted almost to the same extent as the uranium. After two days of leaching about 50% Th is leached from 0.36 to 0.35 mm ore. Thereafter, the content of thorium in solution starts to decline. Later as the tests progress the concentration of thorium in solution again begins to increase steadily. This effect is more noticeable on smaller sized ore.
Precipitate Formation
Whitish flakes are formed in the columns during the early stages of leaching. This precipitate has been identified as mold. As leaching tests progress a second type of a yellow voluminous precipitate forms and is deposited on the surface of the ore particles and on the surface of the columns. This precipitate is insoluble in 1% sulphuric acid and its composition was not investigated. The formation of yellow precipitate is first observed in the columns with the finest ore, these also having the highest concentration of dissolved salts. The salts in the leach solution contained mainly iron, thorium, aluminum, calcium, yttrium and rare earth elements. The polymerization of sulphate complexes is enhanced by the aging of the leached solution and is thought to be the source of the yellow precipitate. Uranium precipitation during both leaching tests was not observed. To prevent or decrease the formation of yellow precipitate, leach solutions with’ high salt contents might be diluted or replaced with fresh lixiviant.
Bacterial Activity
At Agnew Lake, the bacteria become well established after about three weeks. This activity is accompanied by a rapid decrease in the EMF (-380 mV to -700 mV). It was found that during the testwork bacteria became active at various times depending on the size of ore. A high content of dissolved salts in the leach solution and frequent pH changes due to additions of sulphuric acid (primarily for the tests with smaller size of ore) tended to inhibit the growth of bacteria.
Bacteria started to become active after about 4 weeks in tests 1 to 4, and were well established with EMF at -700 mV by 6 weeks. The bacteria did not develop as quickly in tests 5 to 10 and these tests were inoculated with stock bacteria on the 33rd and 49th day of leaching. After 7 to 8 weeks the bacteria became more active and the EMF levelled in a range of -500 to -600 mV. For more details refer to Table 8. The bacteria activity was rapidly improved by diluting the leach solution with 0.5% sulphuric acid, the EMF values then stabilized in a range of -700 to -720 mV.
Sulphuric Acid Consumption
Concentrated sulphuric acid was added during the leaching testwork in order to maintain the pH below 1.9. Test 1 (largest ore) did not require any addition of acid for pH adjustments.
The acid consumption increased with decreasing the size of ore, particularly in the early stages of leaching. Tests employing the smaller size fractions required frequent additions of sulphuric acid. The required additions of sulphuric acid (as 100%), in excess of the 10 kg/t initially introduced to the leaching system for the leaching of 5-zone ore samples, are presented in Table 5.