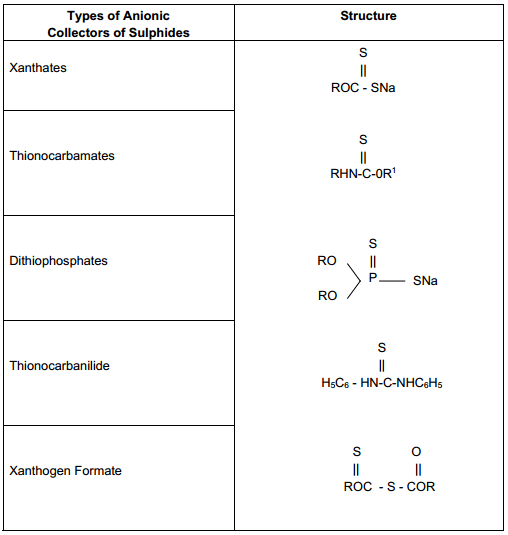
Collectors for sulphide minerals : Anionic collectors are most commonly used for sulphide minerals. By reference to the table (below) it will be note that they are all structurally similar, each having a single sulphur atom double bonded to either a carbon or phrosphorus atom, hence all of the sulphide minerals can be floated with varying success by any of the sulfydric collectors.
Anionic collectors consist of two types according to the structure of the polar group: sulfhydryl type and oxyhydryl type. The term sulfhydryl refers to the SH group present in undissociated form of the collector. The term thiol refers to carbon bonded to the SH. that is, C-SH or R-SH. Both sulfhydryl and thiol (thio) are used to describe this class of collectors. They are widely used in the flotation of sulfide minerals (Avotins et al., 1994). The other anionic type of collectors is oxyhydryl (referring to the OH group), and they arc mainly used in non-sullldc flotation. Compared to the sulfhydryl collectors. the hydrocarbon chain is usually longer. Typically supplied as salts of Na or K. these cations play no role in the action of these anionic collectors.