Subsequent to the work of A. Vita in 1920, a number of investigators have published data dealing with the rapid determination of sulfur in coal using a high-temperature combustion furnace. These, together with several unpublished methods, involve wide differences in technique although they are all similar in principle. .
Materials and Experimental Work
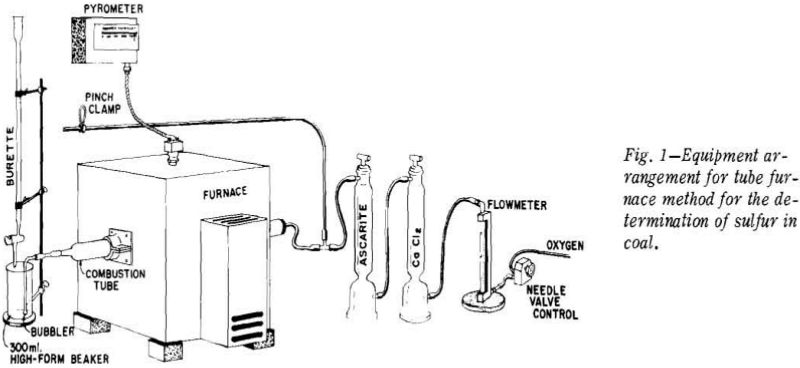
A metered volume of oxygen is passed through calcium chloride for removal of moisture, then through Ascarite for removal of carbon dioxide, and thence through a glass tee to the feed end of the combustion tube. Connected to the side arm of the glass tee is a rubber tube that is closed with a pinch clamp during combustion of a sample. The products of combustion are expelled through a Vycor bubbler, the end of which is submerged 3 cm below the surface of a 1 pct hydrogen peroxide solution contained in a 300 ml high-form beaker. At the completion of the 4- min combustion period the contents of the beaker are titrated to a yellow end point, using a methyl red indicator, with 0.05 N sodium hydroxide (or sodium tetraborate).
Weight of Sample: Several series of tests wherein the sample weight ranged from 0.1 to 0.5 g of -60 mesh coal showed that the lowest variance (standard deviation squared) resulted from the use of 0.5-g samples.
Temperatures: Mott and Wilkinson found that a combination of low temperature (1250°C) and low oxygen flow rate (less than 700 ml per min) tended to give high results due to formation of oxides of nitrogen.
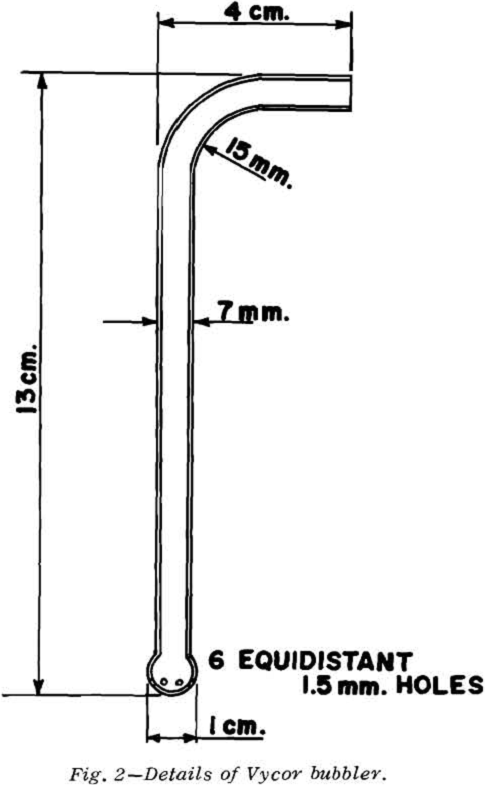
The temperature of the elbow of the bubbler should be at least 75°C to minimize condensation.
Analytical Procedure
Prior to the insertion of the first sample to be a analyzed, the combustion tube should be purged for at least 5 min with purified oxygen at the standard flow rate of 3 to 3 ½ 1 per minute. At the end of the purging period about 100 ml of 1 pct hydrogen peroxide solution and six drops of 0.1 pct methyl red indicator are added to a 300 ml high-form beaker. With the oxygen still flowing through the combustion tube, the bubbler is attached and immersed to a depth of 3 cm in the peroxide solution. Inasmuch as the methyl red indicator was made by dissolving the crystalline substance in sodium hydroxide.
Following the preliminary steps of adjusting the furnace temperature, adjusting the oxygen flow rate, purging the combustion tube, and neutralizing the peroxide solution, the 0.5-g sample contained in a suitable combustion boat is inserted in the combustion tube to a point coinciding with the near edge of the hot zone.
Results and Conclusions
Coals With No Interfering Elements: In an attempt to avoid bias, each of the 40 samples
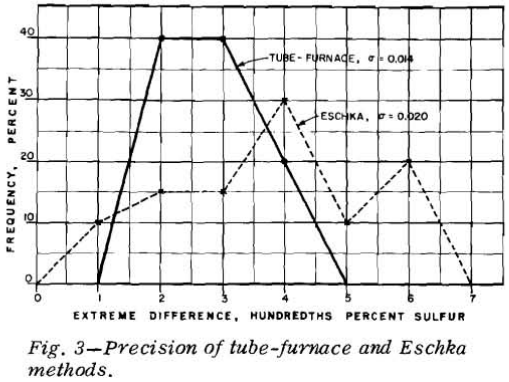
of high-ash raw coal was pulverized to-60 mesh and carefully riffled into two equal parts. These parts were then assigned consecutive sample numbers; that is, samples 1 and 2 were the two halves of the same gross sample, samples 3 and 4 were the two halves of a different gross sample, etc. The analysts were then instructed to determine the sulfur content of the 80 subsamples by both the Eschka and the tube-furnace methods. Several days after the samples were first analyzed they were reanalyzed by both methods. The analysts did not know that pairs of consecutively numbered samples were identical. For 40 pct, or 16 samples, the maximum difference in the four analyses was 0.02 pct and for the same number of samples the maximum difference was 0.03 pct S.
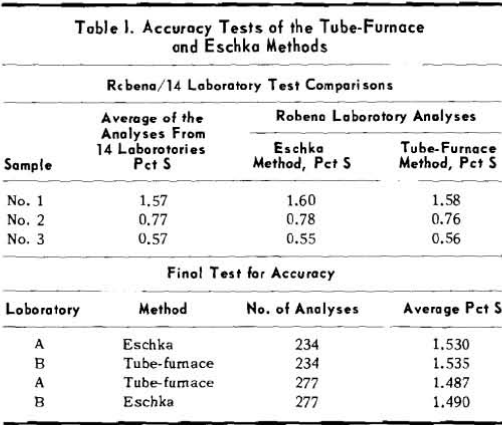
Coals Containing Interfering Elements: Although
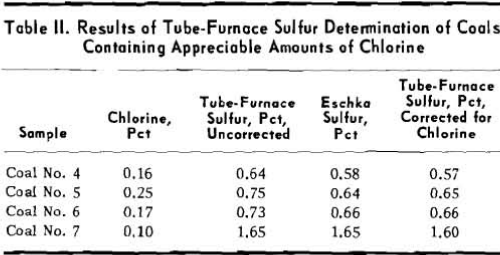
adoption of the tube-furnace method at the Robena Laboratory has greatly reduced analytical costs and delay in reporting the sulfur content of routine control samples of Pittsburgh seam coals, the method has limitations and should not be used indiscriminately. Twenty samples of coal No. 4 contained an average of 0.16 pct chlorine and the tube-furnace sulfur analysis was 0.06 pct higher than the average Eschka analysis. Coal No. 5 was represented by 12 samples with an average chlorine content of 0.25 pct. For this coal the average tube-furnace sulfur was 0.11 pct higher than the Eschka sulfur.

