Table of Contents
- Description of Ore Samples
- Experimental Procedures and Results
- Exploratory Oxidative Leaching Tests
- Oxygen Pressure Leaching
- Base Metal Recovery from Leach Liquor
- Iron-Arsenic Removal by Neutralization and Precipitation
- Copper Recovery by Precipitation With Hydrogen Sulfide
- Zinc Recovery by Solvent Extraction
- Cobalt Recovery by Ion Exchange and Solvent Extraction
A procedure was developed for recovering gold, silver, cobalt, copper, and zinc from a massive sulfide ore by a hydro-metallurgical process. The procedure consists of (1) oxygen pressure leaching; (2) iron, arsenic, and copper removal from the leach solution by precipitation; (3) selective extraction of cobalt and zinc from solution; (4) electro-deposition of the cobalt and zinc from the strip liquor; and (5) cyanidation of the pressure leach residue to recover gold and silver. The results are discussed in terms of efficiency of metal recovery and conclusions drawn as to possible benefits and broad applications of the process.
Introduction
Ores that contain sulfide minerals are important resources for many of these vital commodities. Sulfide minerals are common forms for the strategic and critical metals cobalt and nickel.
Metallurgical processing of sulfide minerals can be complex because the minerals normally contain several associated metals. Cobalt and nickel ores usually contain copper, which can be present in a variety of forms, or other common metals; the cobalt and nickel are typically minor values. Lead, zinc, and copper are often associated with each other. Also, gold and silver are commonly associated with those base metals. Mineral recovery procedures must therefore deal with several metals whose response to processing techniques may be quite different.
When the sulfide minerals are disseminated in a non-sulfide host rock, physical separation is often easily accomplished, and separation of different sulfide minerals from each other may also be possible. The valuable metals are usually recovered as a sulfide mineral concentrate. Some ores, however, are composed almost entirely of sulfide minerals in which small amounts of valuable metals are disseminated in low-value sulfides such as pyrite. These massive sulfide ores do not have a crystalline structure that allows effective physical separation; therefore, chemical recovery methods must be applied to the entire ore. Such processes are both technically and economically difficult for two main reasons. First, concentration of metal values in process streams is low; therefore, large volumes of material must be treated. Second, relatively large amounts of unwanted components, particularly iron and sulfur, are present from which the valuable metals must be separated.
The Turner-Albright deposit in southwestern Oregon is an example of a massive sulfide ore that contains both base and precious metals (Strickler, 1986). Diamond drilling has identified a resource of 3 million tons that grades at 0.05 pct Co, 1.46 pct Cu, 3.32 pct Zn, 3.9 g/t (0.114 oz/st) Au, and 15.2 g/t (0.443 oz/st) Ag (Allen, 1986). A detailed mineralogical study on this material is being conducted at the Bureau’s Albany Research Center in Albany, OR (Mardock et al., 1990). Results to date have shown that the ore consists, in order of abundance, of pyrite, sphalerite, chalcopyrite, marcasite, and trace amounts of tetrahedrite, galena, arsenopyrite, and pyrrhotite. Native gold and cobalt are present in micron- to submicron-size particles that are too small to be processed by conventional beneficiation techniques. The submicron gold particles are dispersed within pyrite, which makes the ore highly refractory to gold recovery by standard cyanidation methods. Cobalt is present only as a substitutional element disseminated in pyrite grains.
We conducted a research program with the goal of developing a procedure to recover the strategic metal cobalt, the other valuable base metals copper and zinc, and the precious metals gold and silver. Studies were primarily focused on the hydrometallurgical treatment of raw ore because of potential environmental problems from roasting techniques with their associated sulfur dioxide emissions; some roast-leach tests, however, were conducted for comparison. The experimental data presented in this report are intended to serve as a starting point for the development of procedures to process complex massive sulfide ores, particularly those containing cobalt, other base metals that are soluble in acid sulfate solutions, and precious metals.
Metallurgical processing of sulfide ores presents problems similar to those found in the treatment of refractory gold ores that contain sulfide minerals and carbonaceous matter. These problems are being addressed at the production level by methods such as pressure oxidation (Jackson, 1982; Berezowsky, 1982). Laboratory tests have demonstrated the feasibility of applying oxidative pressure leaching to base metal sulfides (Dannenberg, 1987; Stanczyk, 1961). Pressure oxidation is therefore a technique that offers promise in recovering both base and precious metals from complex sulfide ores, and industrial processes based on this technique may well be proven competitive for a wide range of applications.
A proposed flowsheet for processing the Turner-Albright ore is shown by the simplified block diagram in figure 1. Major steps in the process are oxygen pressure leaching; precipitation of iron, arsenic, and copper from the leach solution; zinc recovery by solvent extraction and electrolysis; and cobalt recovery by ion exchange, solvent extraction, and electrolysis. Gold and silver are recovered from the leach residue by cyanide or thiourea.
Description of Ore Samples
The raw material used in this study was received at the Salt Lake City Research Center in two components. The first sample, weighing 157 kg (345 lb), consisted of drill core that had been crushed and blended. This composite material was used in most of
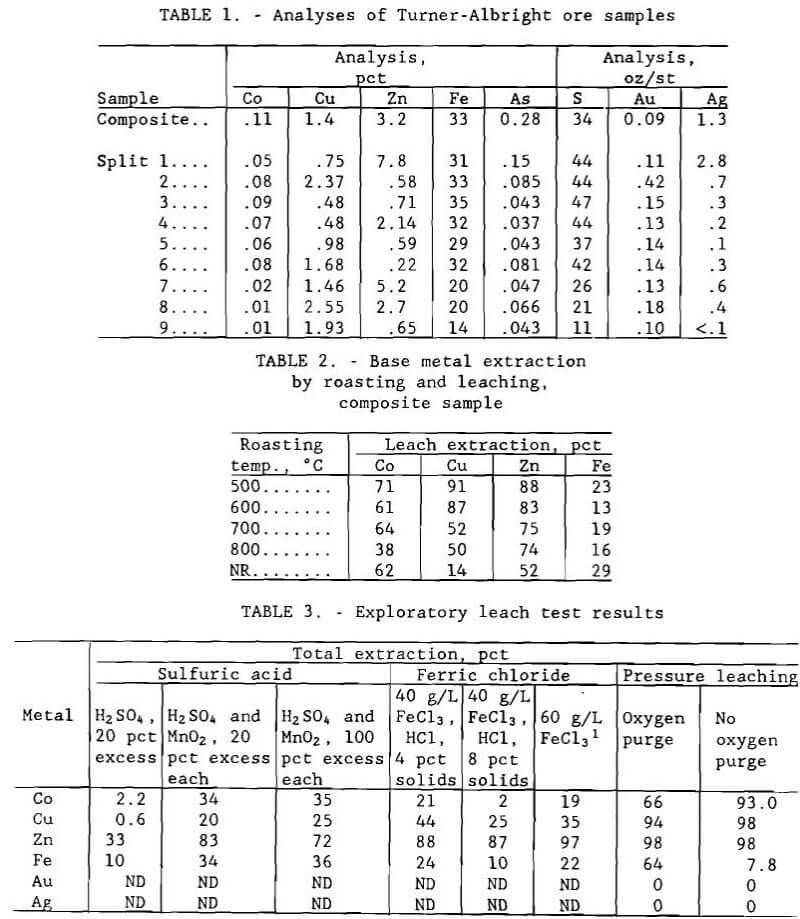
the laboratory tests. The second sample weighed approximately 909 kg (2,000 lb) and consisted of 111 m (360 ft) of half-core plus another quarter of the same core that had been sent by the property owner to a private assay laboratory; the Bureau received what remained of the material after the analyses were completed. The core was composed of nine zones or subzones of ore that had noticeably different composition. Extraction tests were conducted on samples from all nine zones to see if the different materials responded uniformly to test conditions that were optimum for the composite sample. Since the quarter-core assay reject was already crushed, this portion of the core was split into nine samples representing the different zones and used for the tests. Analyses of the composite sample and the nine blended splits are given in table 1; note the high concentrations of iron and sulfur and the presence of arsenic.
Experimental Procedures and Results
Roasting and Leaching
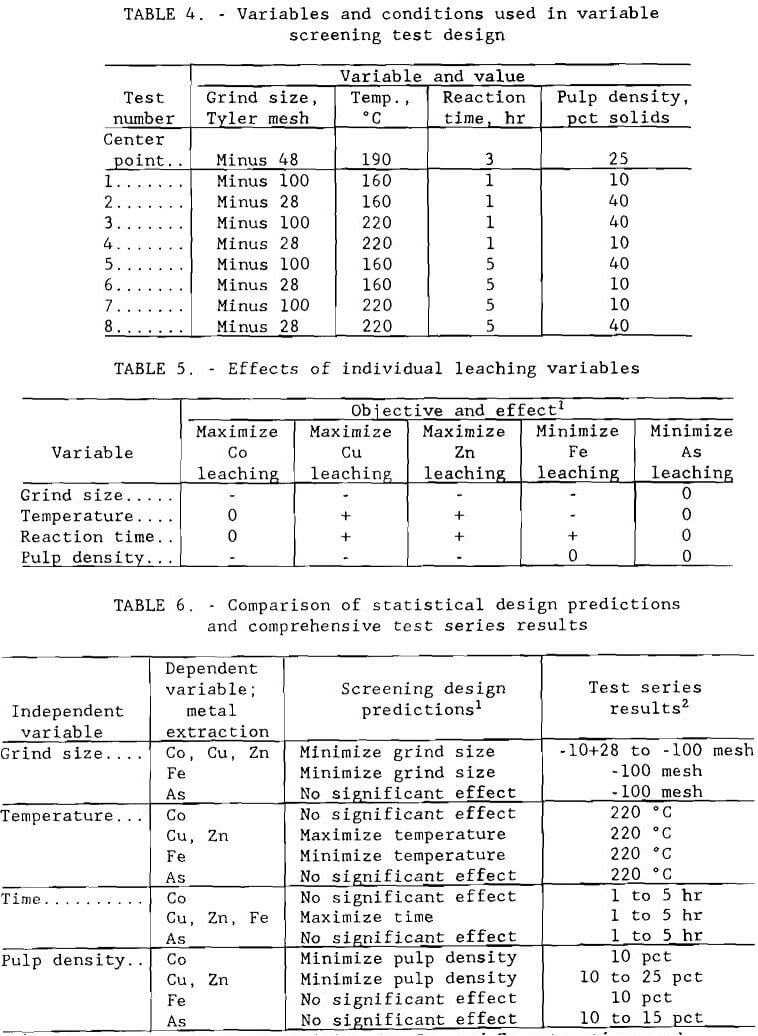
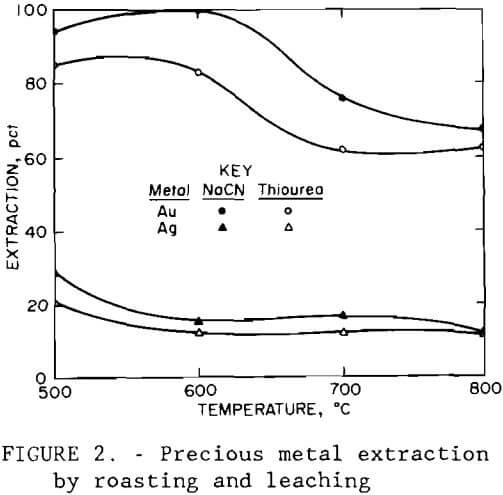
Portions of the composite sample were roasted in a muffle furnace for 5 hr at temperatures of 500° to 800° C (932° to 1472° F). The roasted ore was leached at 20 pct solids and 100° C (212° F) for 2 hr using 50 g/L (100 lb/st) H2SO4, solution. Results are given in table 2, along with those from a test using unroasted ore. Roasting at 500° to 600° C (932° to 1112° F) gave the best cobalt, copper, and zinc extractions.
The roast-leach residues were treated for gold and silver recovery using cyanide solution and thiourea solution. Cyanidation tests were conducted at 20 pct solids with the pH adjusted to 10.5 with lime, and the slurry was agitated for 72 hr at 30° C (86° F) with a sodium cyanide addition of 12 g/L (24 lb/st). Thiourea extraction tests were made with the 20-pct-solids pulp pH adjusted to 1.5 with sulfuric acid, a thiourea addition of 12 g/L (24 lb/st), and the pulp agitated for 3 hr at 30° C (86° F). Two to three hours has been shown by other studies to be sufficient time for thiourea extraction, compared with the much longer time that may be required for cyanidation. Results are shown in figure 2. Again, recoveries were highest for ore roasted at 500° and 600° C (932° and 1112° F). Silver extraction was poor in each case.
Exploratory Oxidative Leaching Tests
Several sulfide ore leaching methods were tested to determine the best base metal extraction technique as a first step in designing a process to recover the base and precious metal values. Leaching reagents used included dilute sulfuric acid, sulfuric acid plus manganese dioxide, ferric chloride plus hydrochloric acid, and oxygen. The composite ore sample was used for these tests.
Dilute sulfuric acid was the first reagent tested. Leach tests were conducted in a stirred, open vessel. Leach time was 5 hr. The acid was added to distilled water and heated to 88° C (190° F) in the reactor. A 50-g charge of minus 100-mesh ore was then added and agitation begun. Stirring speed was approximately 500 rpm. A baseline test was conducted using 20 pct H2SO4 in excess of the amount required to convert all copper, zinc, cobalt, and iron to sulfate form. The next test was identical to the baseline test except that 20 pct excess manganese dioxide was added with the ore; the manganese dioxide requirement was based on the amount needed to oxidize all sulfur from sulfide to sulfate. A dilute sulfuric acid leach using 100 pct excess sulfuric acid in combination with 100 pct excess manganese dioxide was also tested. Ferric chloride leach tests were conducted with pulp densities of 4 and 8 pct solids and hydrochloric acid addition sufficient to lower the pH to 0.5. Leach time ranged from 1.5 to 3 hr.
Results for the tests described above are shown in table 3. Comparing extractions from the sulfuric acid leach tests showed that dilute sulfuric acid alone was not sufficient for leaching to occur, but the addition of 20 pct excess manganese dioxide greatly increased extraction. A further increase in reagent strength to 100 pct excess of each sulfuric acid and manganese dioxide did not significantly increase extraction. Test results from ferric chloride leaching show extractions which were generally comparable to those of sulfuric acid- manganese dioxide leaching with slightly lower iron and cobalt extraction and higher copper and zinc extraction. Extractions increased with decrease in pulp density.
Next, a series of pressure oxidation tests was conducted. Fifty-gram charges of minus 0.15 mm (100-mesh) ore were added with distilled water to a 1-L (0.26 gal) titanium autoclave reactor to make a slurry of 15 pct solids. The temperature was set at 200° C (392° F). After the temperature had stabilized, agitation was begun; oxygen overpressure was increased to a value of 20 to 25 pct in excess of the stoichiometric amount needed to convert all sulfur from sulfide to sulfate, leading to a total pressure of 3171 to 3378 kPa (460 to 490 psig), which was 1723 to 1930 kPa (250 to 280 psi) greater than equilibrium steam pressure. Following the leach, the reactor was immediately quenched in cold water. Filtration was performed, and pH and volume measurements taken at room temperature.
The first pressure leach tests included a 5-min oxygen gas purge before heating the slurry. In subsequent tests, no oxygen purge was used in order to suppress oxidation reactions occurring at temperatures lower than 160° to 190° C (320° to 374° F). Below these temperatures, elemental sulfur is a stable reaction product and tends to coat the unreacted sulfides, thus inhibiting further oxidation.
A comparison of results from the acid and ferric chloride leaches with the autoclave leaches, also given in table 3, shows that oxidative pressure leaching resulted in higher valuable base metal extraction and lower iron extraction than atmospheric acid leaching. Precious metal extractions were effectively zero in pressure leaching. A processing scheme for the Turner-Albright massive sulfide ore was thus developed based on oxygen pressure leaching.
Oxygen Pressure Leaching
Statistical Variable Screening Test Design
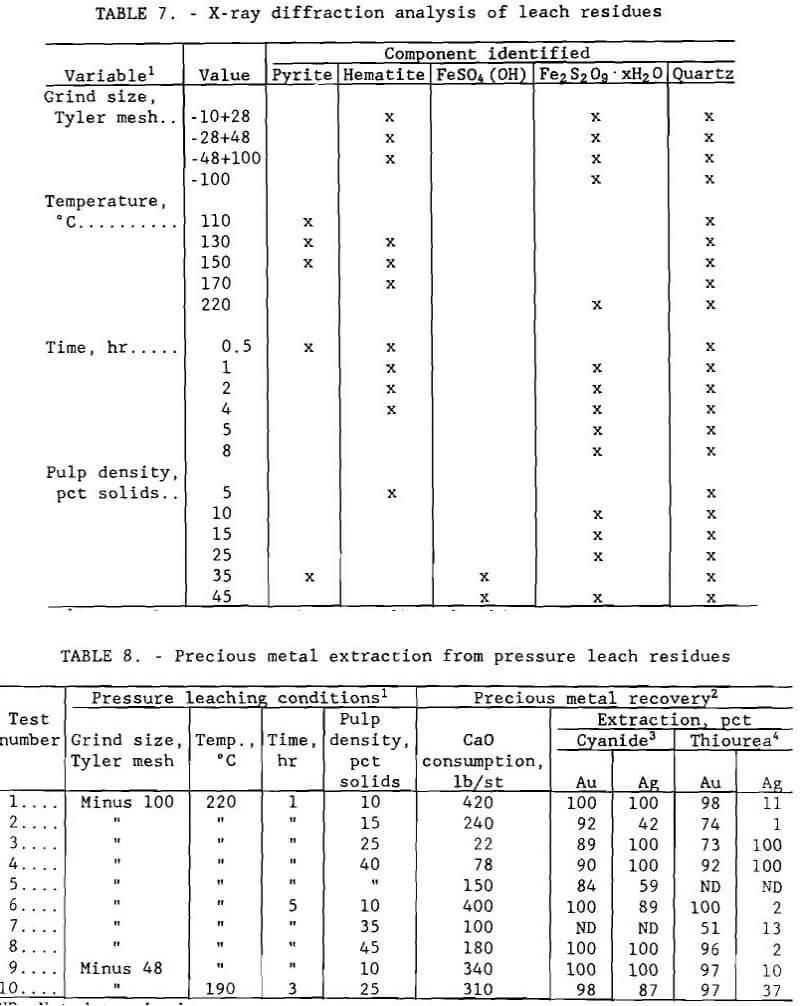
A statistical experimental design was developed to aid in the determination of important variables in oxygen pressure leaching (E.I. duPont de Nemours & Co, 1974). Four variables that are generally considered to be of major importance in pressure leaching were studied: ore grind size, temperature, reaction time, and pulp density. The ranges tested and test conditions are given in table 4. For each test, an entire ore split ground to pass the designated screen was used rather than a size fraction from a larger split. Although oxygen overpressure is also an important factor, it was not included as a separate variable in the design. The oxygen overpressure was maintained at 689 kPa (100 psi) for all tests in this series.
The design used was a half-replicate, eight-test factorial with three center point replicates and a built-in check for variable interactions. Extremely low and extremely high values were used for each variable to enable effects to be easily determined.
Effects of Leaching Variables
Statistical analysis using a t-test was performed on the data to determine the relative importance of the four variables. Effects of the individual variables, shown in table 5, were determined with 95 pct confidence. Metal extractions ranged, in percent, from 3.7 to 76 Co, 5.9 to 99 Cu, 12 to 99 Zn, 1.9 to 73 Fe, and 2.8 to 39 As. Sulfate concentrations ranged from 65 to 305 g/L (130 to 610 lb/st). Conditions that resulted in the highest cobalt, copper, and zinc extraction with the lowest iron and arsenic extraction were minus 0.15 mm (100- mesh) grind size, 220° C (428° F), 5-hr leach time, and 10 pct solids. These conditions resulted in extractions of, in percent, 68 Co, 99 Cu, 98 Zn, 8.9 Fe, and 10 As. The sulfate concentration was 93 g/L (186 lb/st).
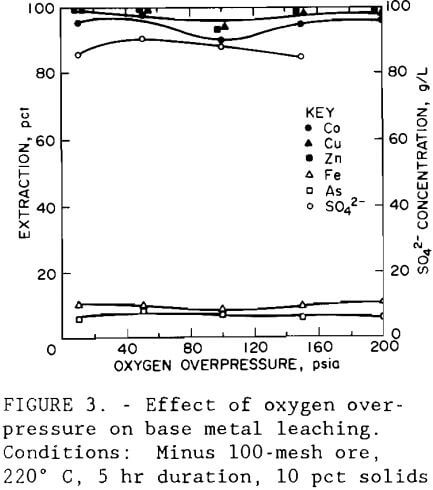
Next, a test series was conducted to determine the effect of oxygen overpressure. Tests were conducted using the conditions established from data obtained in the variable screening design that resulted in the highest valuable metal extraction with the lowest iron and arsenic extraction. Oxygen overpressures tested ranged from 172 to 1379 kPa (25 to 200 psi). Figure 3 shows that regardless of the oxygen overpressure used, extraction of cobalt, copper, and zinc averaged 96, 98, and 98 pct, respectively. Iron and arsenic extractions averaged 9.5 and 6.6 pct, respectively. The sulfate concentration of the leach solution, also shown in figure 3, was found to be independent of oxygen overpressure and had an average value of 87 g/L (174 lb/st).
Additional test series were conducted to establish trends for the effects of grind size, temperature, leach time, and pulp density. These tests were designed to generate data to confirm or refute the optimum conditions predicted by the variable screening design. The oxygen overpressure was maintained at 689 kPa (100 psi) for all tests. Tests were conducted in random order to eliminate trends caused by test procedure or experimental error, and one to two duplicate tests were conducted in each series. Test conditions were maintained as given above except for the variable being tested.
Results are illustrated in figures 4 through 7. Averages of replicate tests were used. Referring to figure 7, the discontinuity in extraction at 35 pct solids pulp density resulted from the inability to obtain reproducible results at pulp densities greater than 25 pct solids. The variable effects predicted for maximum cobalt, copper, and zinc extraction with minimum iron and arsenic extraction from the statistical variable screening design are compared in table 6 with the results from the more comprehensive test series.
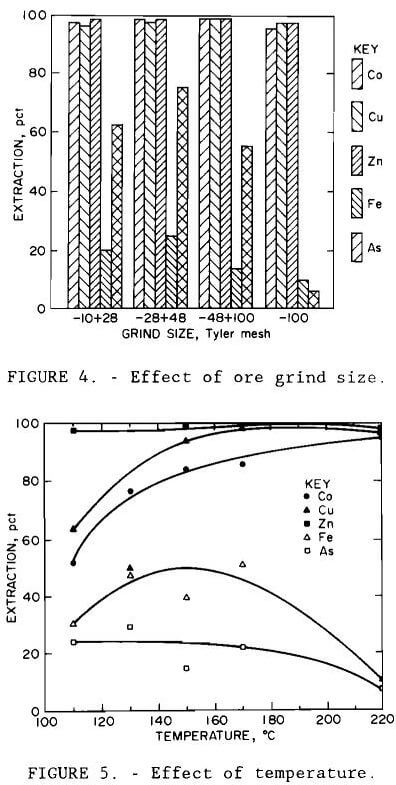
Results from these test series concurred with the predictions from the variable screening design, with the exception of grind size, whose influence was not as great as predicted. The optimum conditions determined by the test series were minus 0.15 mm (100-mesh) grind size, 220° C (428° F), 1- to 5-hr leach time, 10 pct solids, and 172 to 689 kPa (25 to 100 psi) oxygen overpressure.
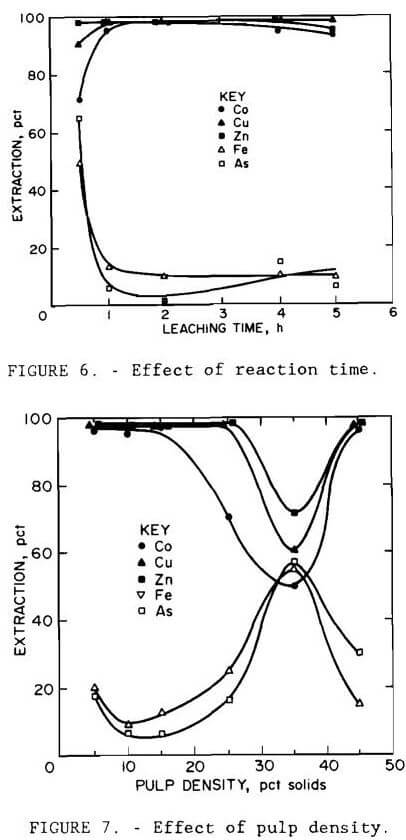
Under all conditions tested, extractions of cobalt, copper, and zinc paralleled each other, as did those of iron and arsenic. Except for the tests conducted at 35 and 45 pct solids and those at 170° C (338° F), replication of results was within 10 pct. The error in replication for the tests conducted at 170° C (338° F) occurred only for arsenic, where extraction varied by 40 pct. Since duplicate results for the other metals varied less than 8 pct, the arsenic variation was probably due to assay errors.
Composition of Leach Residue
Analysis by X-ray diffraction of the residues showed that the iron sulfide present in the ore was oxidized and hydrolyzed to an iron sulfate hydrate of composition Fe2S2O9·xH2O, an intermediate hydrolysis product that can be further hydrolyzed to a material similar to jarosite, KFe3(SO4)2(OH)6. Jarosite – like compounds, which will be designated as pseudo-jarosite, can be formed with monovalent cations other than potassium. Products are formed according to the following order of reactions:

Arsenic can precipitate as ferric arsenate, FeAsO4·2H2O. When these reactions are allowed to occur, the iron and arsenic precipitate, thus reducing their concentration in the leach solution. If the hydrolysis goes completely to pseudo-jarosite, the residue becomes refractory to silver recovery by cyanidation.
Complete residue analyses for all experiments are summarized in table 7. Quartz, a barren component of the ore, is inert to the hydrometallurgical treatment examined in this research study.
The amount of iron precipitated increased with temperature and time, decreased with pulp density, and was unaffected by grind size. Any arsenic present was oxidized and precipitated with the iron. Consideration must therefore be given to the composition of the leach residue as well as variable interactions for oxidative pressure leaching of the Turner-Albright ore. Conditions which resulted in maximum cobalt, copper, and zinc extraction, minimum iron and arsenic extraction, and a residue of composition Fe2S2O9·xH2O were minus 0.15 mm (100-mesh) grind size, 220° C (428° F), 1- to 5-hr leach time, 10 pct solids, and 172 to 1379 kPa (25 to 200 psi) oxygen overpressure.
Precious Metal Recovery from Leach Residue
Residues from pressure leach tests were used in experiments to compare the extractions of gold and silver using cyanide and thiourea. Gold and silver leach tests could not be conducted on every residue because of insufficient residue sample size. Those residues of adequate size were tested in random order. Two grab samples were taken from each and placed in two open 400-ml beakers. Water was added to bring the pulp density to 20 pct solids, and each pulp was stirred by an impeller. The pH of the pulp to be cyanided was measured, then lime was added to raise the pH above 10. During the test, more lime was added as needed to maintain this pH. Total lime consumption was determined at the end of each test. After the pH of the sample stabilized, 10 g/L (20 lb/st) sodium cyanide was added. Similarly, 10 g/L (20 lb/st) of thiourea was added to the other sample. A potential advantage of using thiourea is that acid residues do not have to be neutralized, a procedure that can require large amounts of lime. Tests were operated for 72 hr with water addition as needed to maintain the pulp density. After leaching, each sample was filtered and washed, and the leach residue was air dried.
Gold and silver extractions were computed using calculated head assays. Results given in table 8 show that, in general, high gold and silver extractions were achieved using cyanide regardless of the pressure leach conditions used. High gold extraction was also attained with thiourea.
However, silver extraction using thiourea tended to be low. This result can be explained by the fact that thiourea decomposition products can precipitate silver; gold is not affected.
Tests Using Individual Core Splits
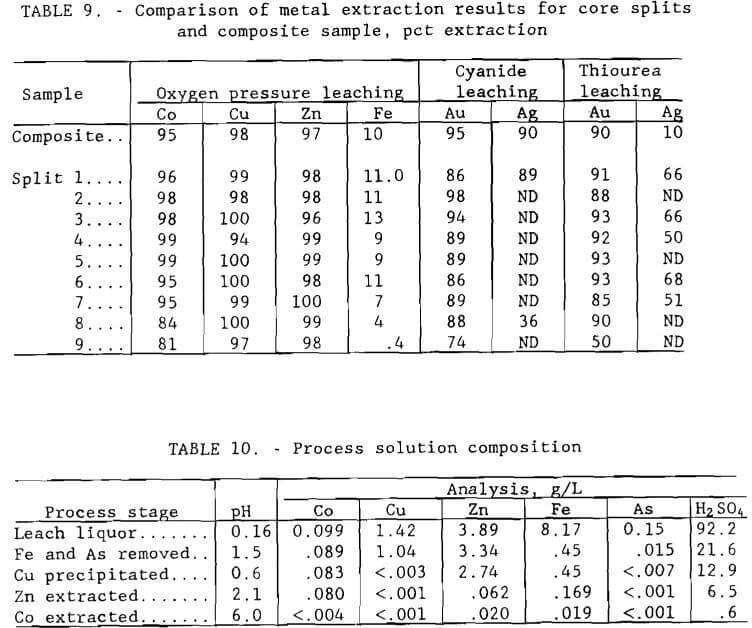
Samples from the nine core splits were tested for base and precious metal recovery using conditions determined to be effective for the composite sample in order to determine if differences in ore composition could be accommodated by the proposed extraction procedure. Results for the splits are compared in table 9 with those for the composite material. The splits were pressure leached at minus 0.15 mm (100-mesh) grind, 220° C (428° F), 5-hr duration, 10 pct solids, and 689 kPa (100 psi) oxygen pressure. Cyanide leaching conditions were pH 10.5, 12 g/L (24 lb/st) NaCN, 30° C, 72 hr, and 20 pct solids; thiourea leaching conditions were the same except for pH 1.5 and 2 hr duration. Cyanide and thiourea leaching tests were conducted in bottles with rolling agitation exposed to the atmosphere. Extraction values for the composite sample are nominal results from the series of tests discussed above. Results of the tests show that the proposed metallurgical treatment will not be adversely affected by changes in process feed.
Base Metal Recovery from Leach Liquor
The scheme illustrated in figure 1 was investigated for recovering the valuable base metals from the leach solution. For reference, table 10 shows how the process solution composition changed through the stages of the recovery procedure.
Iron-Arsenic Removal by Neutralization and Precipitation
Iron, a major contaminant in the base metal recovery process, was removed from the composite leach solution along with arsenic by precipitation as pseudo-jarosite (Dannenberg et al., 1987). Conditions for selective removal of iron and arsenic by precipitation were determined by conducting a series of tests in a three-neck distillation flask equipped with a water-cooled condenser and air sparger. A heating mantle was used to heat the distilling flask up to 95° C (203° F). The apparatus used in this experiment is shown in figure 8. Best results were achieved by adding 3 g/L (6 lb/st) sodium peroxide to oxidize ferrous iron to ferric iron and then neutralizing the solution with sodium hydroxide to pH 1.5. Sufficient sodium was added by this procedure to convert all iron in solution to sodium jarosite, NaFe3(SO4)2(OH)6. Ninety to 95 pct of the iron and arsenic from a composite leach solution containing, in grams per liter, 0.099 Co, 1.42 Cu, 3.89 Zn, 8.17 Fe, and 0.15 As were precipitated, and approximately 10 pct Co, 27 pct Cu, and 14 pct Zn were coprecipitated. The precipitated product was identified by X-ray diffraction to be a jarosite.

Copper Recovery by Precipitation With Hydrogen Sulfide
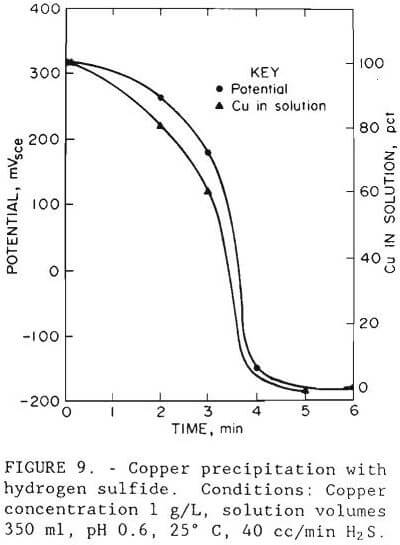
A simple and rapid technique for removing copper from solution is to precipitate it with hydrogen sulfide. Initial precipitation tests were carried out in a 1,000-ml (0.26 gal) glass beaker using synthetic solution. Hydrogen sulfide gas was bubbled through 350 ml (0.09 gal) of test solution that contained 1 g/L (2 lb/st) Cu as copper sulfate at a rate of 40 cc/min (2.4 cu in/mm). The solution pH was adjusted from 1.5 to below 1 with sulfuric acid, the procedure that would be used in the actual process to minimize coprecipitation of cobalt and zinc. The end point of the precipitation reaction was detected by measuring the oxidation-reduction potential. The precipitation curve for a typical test is shown in figure 9. The amount removed in less than 6 min at 25° C and pH 0.6 was 99.9 pct at minus 150 mVSCE.
Results using iron-free leach liquor, shown in figure 10, show that copper was selectively precipitated in 8 min at pH 0.7. The apparatus shown in figure 8 was used in the experiment. The filtrate from the iron removal step contained, in grams per liter, 0.089 Co, 1.04 Cu, 3.34 Zn, 0.45 Fe, and 0.015 As. Addition of hydrogen sulfide precipitated 99.7 pct Cu, 6.3 pct Co, 18.0 pct Zn, and more than 53 pct of the remaining arsenic. Filterability of the sulfide product was good.
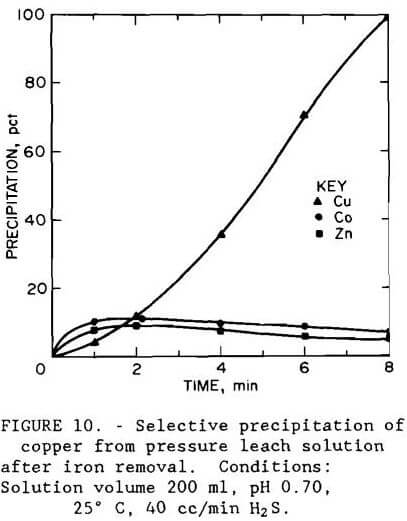
Precipitation as cupric sulfide was not an effective method for recovering copper unless iron and arsenic were removed from solution first since these elements coprecipitated with the copper.
Zinc Recovery by Solvent Extraction
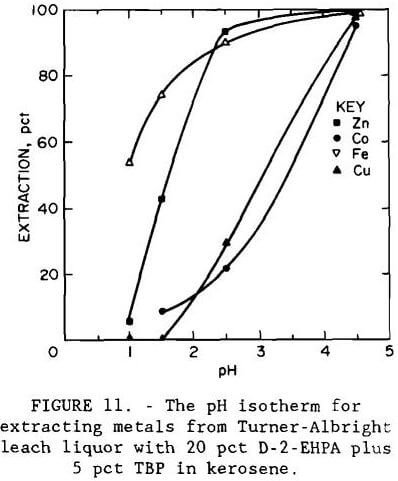
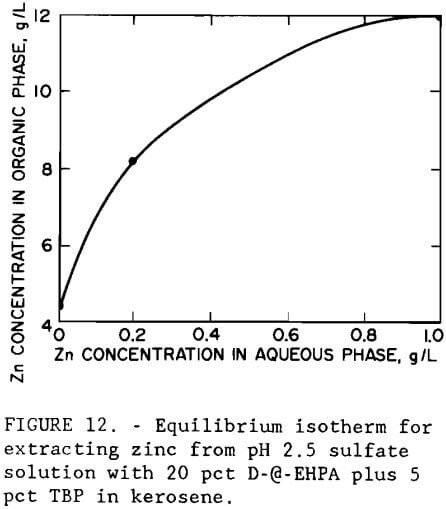
A series of batch shaker tests was conducted to investigate the effect of pH on metal extraction by an organic phosphoric acid. The extractant was a mixture of 20 vol pct D-2-EHPA with 5 vol pct tributyl phosphate (TBP) in kerosene. The experimental pH isotherm, shown in figure 11, indicates that iron is extracted before zinc; therefore, it should be removed prior to solvent extraction. The isotherm was developed by making shaker tests with the iron- and arsenic-containing leach liquor using an organic-to-aqueous (O:A) phase ratio of 1. At pH 2.5, the organic extractant showed greater selectivity for zinc than for the remaining metals. Increasing the pH to 4.5 resulted in the extraction of copper and cobalt. Figure 12 shows the experimental equilibrium isotherm for the extraction of zinc from a sulfate solution at pH 2.5. Maximum loading capacity for the extractant was 12 g/L (24 lb/st) Zn. Application of the McCabe-Thiele method (McCabe et al., 1976) to the isotherm showed that only one extraction stage at an O:A ratio of 1 would be required to produce a raffinate of less than 0.06 g/L (0.12 lb/st) Zn from a feed containing 2.7 g/L (5.4 lb/st) Zn.
Additional shaker tests showed that the loaded D-2-EHPA extractant could be stripped from 3.87 to 0.075 g/L (7.74 to 0.15 lb/st) Zn in a single stage with 98 g/L (196 lb/st) H2SO4 solution using an O:A ratio of 20.
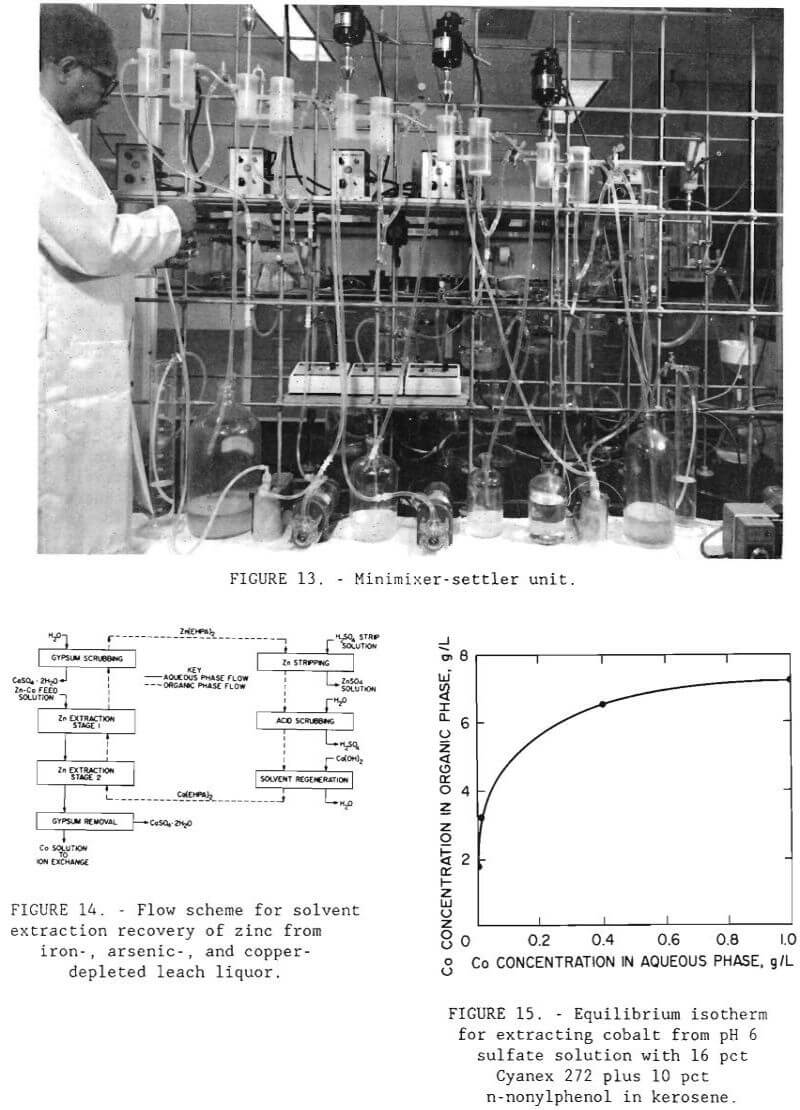
Filtrate from the copper sulfide precipitation step containing, in grams per liter, 0.083 Co, <.003 Cu, 2.74 Zn, 0.450 Fe, and <.007 As was treated by a continuous solvent-extraction operation to recover the zinc. A minimixer- settler unit (figure 13) was operated for seven complete cycles. The flow scheme for the operation is shown in figure 14. The unit was made up of 2 extraction stages, 2 scrubbing stages, 1 stripping stage, and 1 solvent regeneration stage. The calcium form of D-2-EHPA was used for extraction. The O:A phase ratio for extraction was 1. The raffinate contained, in grams per liter, 0.080 Co, <.001 Cu, 0.062 Zn, 0.169 Fe, and <.001 As. The gypsum scrubbing stage was effective in removing physically entrained calcium sulfate that otherwise would be carried over to the strip circuit. The loaded organic was stripped with 98 g/L (196 lb/st) H2SO4 solution at an O:A ratio of 10 to produce a pregnant electrolyte that contained 33.4 g/L (66.8 lb/st) Zn. The O:A ratio of 10 was used because flow rates were easier to control in the system than those for a higher O:A ratio. The spent organic was regenerated by first scrubbing off excess acid and then contacting with 8 g/L (16 lb/st) Ca(OH)2 solution at an O:A ratio of 1.
The pregnant electrolyte was pumped through activated carbon to remove trace amounts of organics and then fed to a 1-L glass electrolysis cell fitted with an aluminum cathode and two lead oxide-coated titanium anodes (Smith et al., 1979). The cell was operated at 882 A/m² (82 A/ft²) producing a product that assayed 96.3 pct Zn. Impurities were mainly lead (0.5 pct), copper (0.04 pct), cobalt (0.03 pct), and iron (0.015 pct). Current efficiency was 69 pct.
Cobalt Recovery by Ion Exchange and Solvent Extraction
Solution exiting the zinc recovery procedure contained, in parts per million, 80 Co, 169 Fe, 62 Zn, <1 Cu, <1 As, 256 Mg, 25 Mn, and traces of Pb and Ni. Up to this point in the process, the presence of the last four of these elements had not been of concern, although, as seen in the previous section, some lead appeared in the zinc product. It was decided to recover cobalt by first upgrading its concentration in solution by ion exchange, then selectively removing it and further concentrating it by solvent extraction. The ion- exchange process is usually not completely selective, particularly when other cations are also present in approximately the same concentration; therefore, it was expected that impurity metals would be extracted along with the cobalt.
Before the ion-exchange experiments were begun, shaker tests were conducted to determine the feasibility of using an organic phosphinic acid in the solvent-extraction procedure. The extractant was a mixture of 16 vol pct Cyanex 272 with 10 vol pct n-nonylphenol as a modifier in a kerosene diluent. Figure 15 shows the experimental isotherm for this extractant. The maximum loading capacity was found to be 7.2 g/L (15.4 lb/st) Co. In applying the McCabe- Thiele method to the extraction isotherm, more than 99 pct of the cobalt should be recoverable from a solution containing. 1 g/L (2 lb/st) Co at pH 6 in a single stage at an O:A phase ratio of 1.
Upon completion of the shaker tests, efforts were directed toward concentrating the cobalt to approximately 1 g/L (2 lb st). The zinc raffinate was neutralized to pH 3 with sodium hydroxide, and approximately 50 bed volumes (BV) were pumped downward at a rate of 150 ml/min (3 gpm/ft²) through a 3.8-cm- (1.5-in) diam column filled with 500 ml of Dow 4195 cation exchange resin. This resin has a high selectivity for cobalt over nickel and also rejects magnesium and manganese (Jeffers et al. , 1985). Cobalt was effectively extracted; the column effluent contained less than 0.004 g/L (4 ppm) Co. The loaded column was eluted with 4 BV of 20 g/L (40 lb/st) H2SO4 solution to recover the cobalt. The pregnant eluate from the column contained 1.0 g/L (2 lb/st) Co plus, in parts per million, <11 Cu, 61 Zn, 51 Fe, 64 As, and <35 Ni.
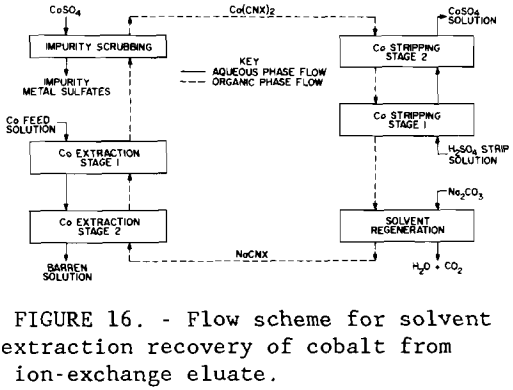
The eluate was further purified and concentrated in a multiple – stage solvent-extraction unit shown in figure 16. A circuit containing 2 extraction stages, 1 scrubbing stage, 2 stripping stages, and 1 solvent regeneration stage was operated using the Cyanex 272 extractant. The pH of the aqueous feed was adjusted to 6 with sodium hydroxide. The flow rate of the organic solution, aqueous feed, scrubbing liquor, and regeneration liquor was 10 ml/min (0.003 gal/min) each; the flow rate of the stripping liquor was 1 ml/min (0.0003 gal/min). In the scrubbing stage, the loaded solvent was contacted with 7.0 g/L Co sulfate solution, the highly concentrated cobalt crowding off impurity metals. Loaded Cyanex 272 was stripped with 49 g/L (98 lb/st) H2SO4 solution, and the spent organic was regenerated to its sodium form with an 8-pct Na2CO3 solution and returned to the extraction circuit. Raffinate from the solvent-extraction circuit analyzed, in parts per million, 1 Co, 1 Zn, 2 Cu, 1 As, and 3 Ni. Cobalt was electrowon from the strip solution containing 26.8 g/L (53.6 lb/st) Co using two PbO2 -Ti anodes and a stainless-steel cathode blank at 50° C (122° F). Current efficiency was 90 pet at a current density of 44lA/m² (41 A/ft²). Analysis by X-ray fluorescence showed that the major impurities in the metal product were lead (approximately 2 pct), zinc (1 pct), and iron (1 pct).
Conclusions
A procedure based on oxidative pressure leaching was shown to effectively recover both base and precious metals from the Turner-Albright massive sulfide ore. Pressure leaching with oxygen extracted 95 pct or more cobalt, copper, and zinc. Iron and arsenic were effectively removed from the leach liquor by jarosite precipitation. Hydrogen sulfide precipitation removed more than 99 pct of the dissolved copper. The resulting liquor was processed by solvent extraction, ion exchange, and electrolysis to recover 95 pct or more of the cobalt and zinc. Cyanide leaching of the pressure leach residue recovered more than 90 pct of the gold and silver. The hydrometallurgical process offers advantages over roast-leach procedures, effectively eliminating problems with sulfur dioxide emission and arsenic-containing effluent solutions.