Chemical Processes-Pigment Grade Titanium Dioxide by Hydrochloric Acid Leaching
The following reactions give the chemistry of this process:
CaTiO3 + 4HCl = TiOCl2 + CaCl2 + 2H2O
The titanium is in solution as TiOCl2. It is then hydrolyzed to TiO2 as follows:
TiOCl2 + H2O = TiO2 +2HCl
The hydrolysis is brought about by dilution of the acid, heat and seeding with TiO2.
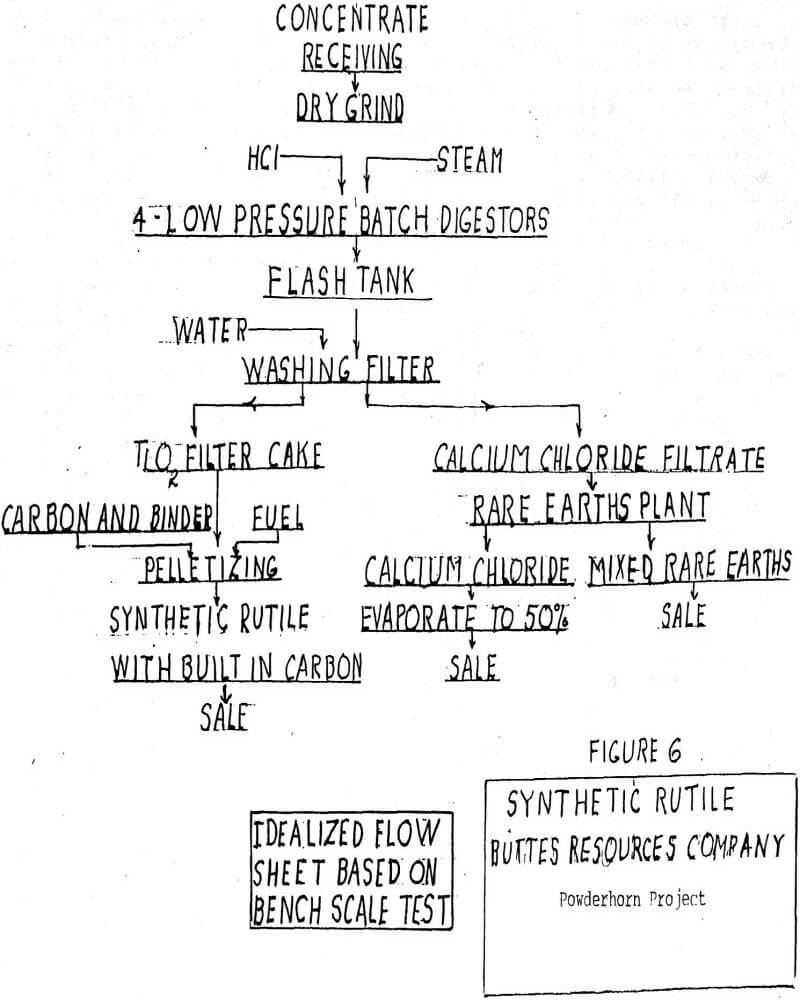
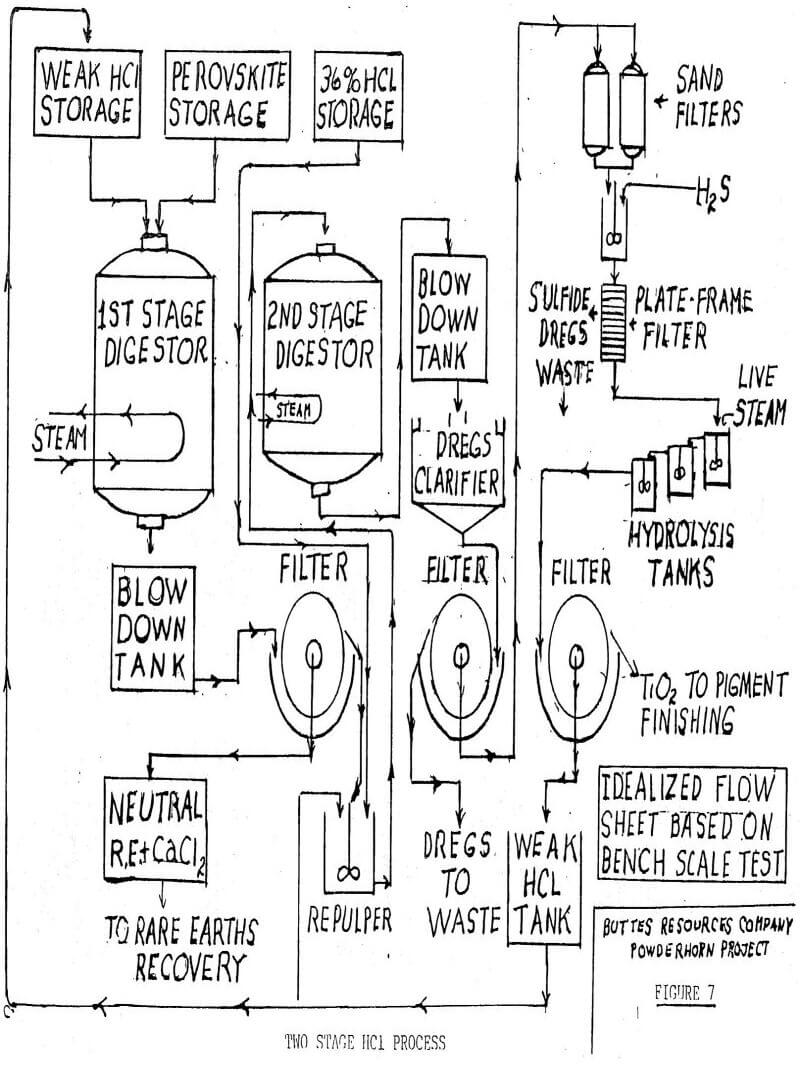
Because the titanium goes into solution, it can be filtered away from augite dregs and purified to remove traces of base metals. Figure 7 gives a hypothetical and idealized flow sheet for a two-stage plant to produce pigment grade titanium dioxide and rare earths. Each of the major steps in the process has been demonstrated at the bench scale level but there has been no continuous pilot plant work. In the first stage perovskite is digested with returning HCl from the hydrolysis step following the second stage.
After the calcium chloride has been stripped of rare earths there are two alternates for its disposal. First is the possibility of evaporation to a 50% solution and sale to the chemical company that supplied the HCl. No one expects an even tradeoff, but it could move out in the same tank cars that brought in the HCl. The second alternative is to also evaporate to 50% and treat with sulfuric acid to regenerate HCl. Other than the complexity of the process, it has the disadvantage of producing a solid waste of calcium sulfate.
The second digester stage carries the process to completion with the titanium in solution as titanium oxychloride. The flow sheet does not show pigment finishing, but this involves calcining, regrinding and washing with final grinding. Any chloride process produces the rutile modification of titanium pigment, and this is the most desirable form.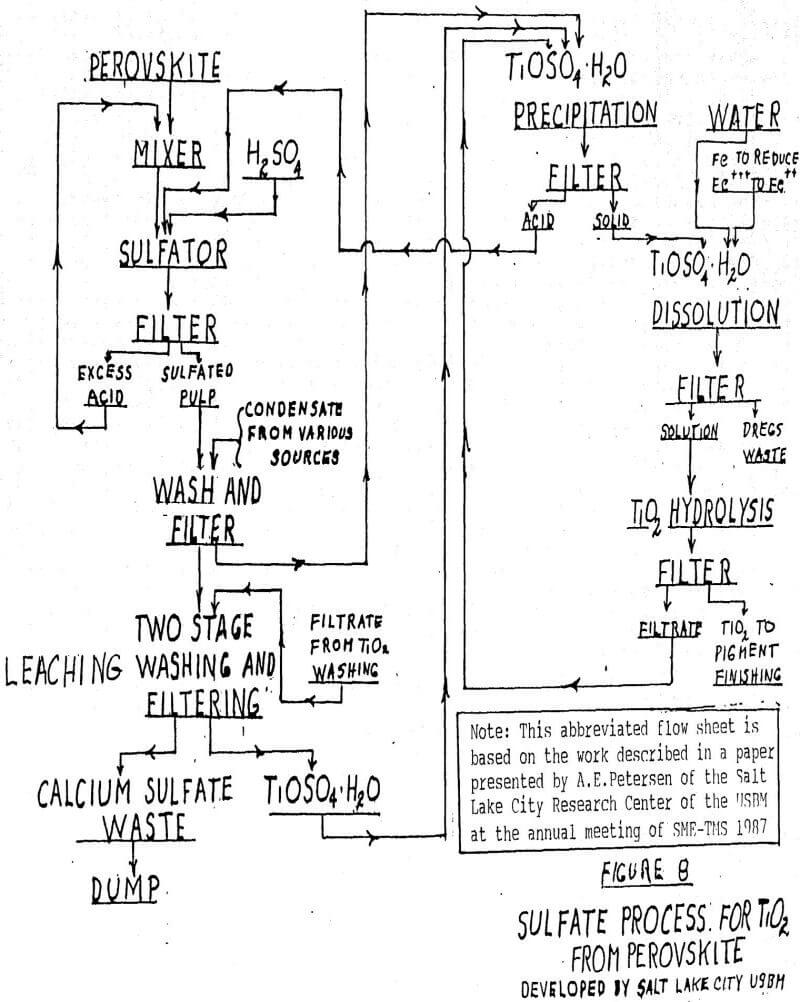
Chemical Processes-Pigment Grade Titanium Dioxide and Rare Earths By the Sulfate Process
This process has been developed at the bench scale level by the United States Bureau of Mines, Salt Lake City Station. At the SME-TMS meeting in 1987, A. E. Petersen gave a paper on preparation of pigment grade titanium dioxide from Powderhorn perovskite by a sulfuric acid process, and C. F. Davidson and J. K. Winters gave a paper on the recovery of rare earths from the leach liquors of the sulfate process. These papers presented rather complex flow sheets. In this paper we have attempted to simplify these flow sheets without losing the general chemistry involved. The following is an oversimplification of the chemistry involved.
CaTiO3 + H2SO4 = TiOSO4 + CaSO4 + 2H2O
TiOSO4 + H2O = TiO2 + H2SO4
The titanium dioxide produced is made into finished pigment in much the same way as is done in conventional sulfate pigment plants. See Figure 8 for simplified flow sheet.
Titanium Carbide as a Synthetic Rutile
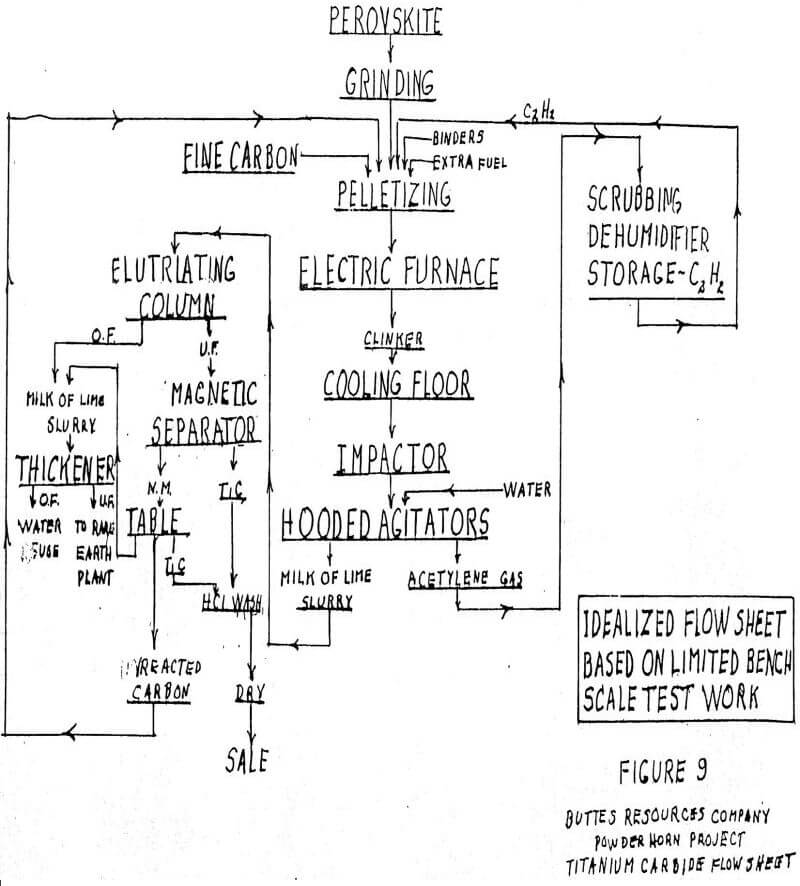
The USBM has demonstrated at the bench scale level that titanium carbide (TiC) can be made from perovskite. Titanium carbide contains 80% Ti, whereas pure rutile contains only 60% Ti. Therefore, on a 100% recovery basis TiC will make 33% more pigment than natural rutile. The process used at Albany involved electric furnace treatment of perovskite with carbon. The temperature was in the range of that required for calcium carbide, i.e., +-1500 C. The pot furnace used was quite small. The mass in the furnace does not melt to a liquid state and only a clinker is formed. The chemistry is as follows:
CaTiO3 + 6C = TiC + CaC2 + 3CO
Slaked with water, only the calcium carbide reacts, releasing acetylene and producing a milk of lime slurry containing titanium carbide crystals of rather uniform size of about .5 mm
The crystals can be recovered by magnetic separation and tabling. (It is not clear why they are magnetic.) Samples of the carbide have been chlorinated in a tube furnace at low temperature. Once started, the reaction is exothermic. The chemistry is believed to be:
TiC + 2Cl2 + O2 = TiCl4 + CO2
In the chloride process for titanium pigments the chemistry is:
TiCl4 + O2 = TiO2 + 2Cl2
The use of pure oxygen regenerates chlorine in a high state of purity. The following reaction for the chlorination of natural rutile will demonstrate the advantage of titanium carbide with its built-in carbon:
TiO2 + 2C + O2 + 2Cl2 = TiCl4 + 2CO2
In the above reaction, carbon must be added, whereas with titanium carbide no extra carbon is needed.
The advantages of titanium carbide as a substitute for natural rutile are:
- Chlorinates at low temperature and exothermically, which reduces carryover of impurities and may lower energy requirements.
- Produces a theoretical 33% more pigment and would have a lower per kg of pigment, raw material freight cost.
- Requires no added carbon in the chlorination process.
Of course, it is yet to be proven that titanium carbide can be produced at a price competitive with natural rutile.
In the process the rare earth reports in the milk of lime slurry and could be worked up by converting them to chlorides.
We have speculated that a commercial plant (see Figure 9 for flow sheet) might start with a shaft furnace to pelletize perovskite mixed with carbon. At least part of the fuel for the furnace would be provided by the acetylene from the slaking operation. The furnaces would operate in a neutral atmosphere to reduce the oxidation of the carbon. Only bonding is intended and no reaction is expected.
The shaft furnace operates across an aisle from a companion electric furnace. There might be four pelletizing and four electric furnaces in a model plant for study.
The electric furnaces would be stationary with roll-out bottoms. The bottoms would be rather shallow, inasmuch as heat must penetrate a non-fluid mass without benefit of circulation. The cycle starts with a bottom on holding fire. It is rolled under the pelletizing furnace and receives a charge. The hot charge is rolled under the electric furnace and pushed up into a tight seal with the furnace bottom by hydraulic jacks. The heat may take several hours. When finished, the bottom is lowered, rolled out, picked up by overhead crane and moved to the end of the aisle, where it is rotated on trunions and dumped onto the chill floor. After colling, it is dozed through an impactor and into a hooded, negative pressure set of agitators. The hoods collect the acetylene, which is sent to a dehumidifying system before being used in the pelletizing furnaces.
The milk of lime slurry is processed to recover the titanium carbide by various magnetic, gravity and elutriation methods. It is given a final HCl wash to reduce lime to a minimum. The washings pass to the rare earths section, which recovers rare earths by a hydrochloric acid process.
We do not know exactly what the power requirements would be because the furnace used at Albany was too small for good instrumentation. It has been speculated that the requirement might be of the order of 8. kwh per kg of product, when starting with a red hot charge. Some of the pre-heat may have to come from outside sources of energy. The four electric furnaces might be 15 kva, and an ideal situation would be captive over the fence power from a plant hard by a captive coal mine.
The sulfate process is the clear choice for pigment production. It uses a cheaper, less corrosive acid, and would require a less expensive plant. It has the disadvantage of producing a solid waste (presuming that the calcium chloride produced in the chloride process can be sold) . Also, it produces an anatase pigment in an industry increasingly committed to the rutile type of pigment.
 |
 |
 |
 |
 |
 |

