Table of Contents
The development of a fused-salt Titanium Electrorefining technique to recover hyperpurity titanium from offgrade sponge and mill scrap, under investigation by the Federal Bureau of Mines Electrometallurgical Experimental Station at Boulder City, Nev., for several years has been described in many publications. This report describes the investigations to obtain greater capacity by increasing the depth of cathode immersion for deposition.
The first electrorefining investigations were performed in a 12-inch-diameter cell at a maximum bath depth of 14 inches, which permitted a cathode-immersion depth of up to 8 inches. This depth restriction was necessary to avoid contact between the cathode deposit and the anode titanium scrap in the bottom of the cell, resulting in an electrical short. The capacity of this cell was increased by enlarging its top opening to a 16- by 32-inch rectangle and providing three additional cathode-withdrawal ports.
Various operating techniques were studied, using this cell, but it did not offer a feasible cell design for commercial adaptation, and a new cell was made to overcome some of its inadequacies and those of the earlier cells.
Equipment
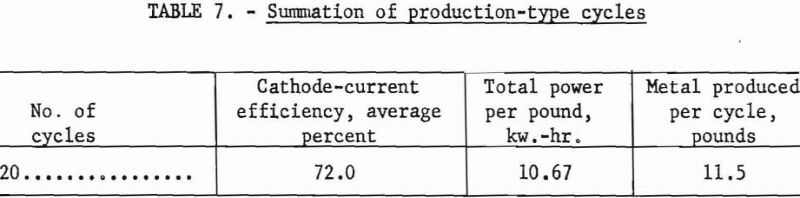
The cell for these investigations was constructed by modifying a vacuum-distillation furnace and retort, which had been used for purifying Kroll sponge by replacing the water-cooled head with a 10-inch gate valve and receiving chamber. The cell, shown in figure 1, was 27 inches in diameter and 60 inches deep and could hold up to 41 inches or 1,400 pounds of electrolyte. This depth of electrolyte allowed cathode immersions of up to 30 inches.
The scrap titanium used as the anode in these investigations was placed in the bottom of the cell.
The furnace used for maintaining the cell at operating temperature was a standard nichrome-grid pit-type furnace with a 40-kw. rating.
The deposition current was supplied from copper oxide rectifiers equipped with continuous controls to provide regulation of d.c. amperage from 0 to 1,800 at voltages of 0 to 5.4.
Electrolyte Preparation and Cell Additions
After the assembled cell had been thoroughly tested for leaks and could maintain 26.3 inches of vacuum for 24 hours, 1,000 pounds of anhydrous sodium chloride was added to the cold cell. To this was also added 100 pounds of offgrade titanium sponge and 390 pounds of “master mix”. The master mix provided enough soluble titanium to produce an electrolyte containing approximately 5 percent soluble titanium. The master mix was prepared by reacting the stoichiometric quantities of sodium and titanic chloride to produce a mixture of sodium chloride and 20 percent soluble titanium dichloride expressed as titanium. A flow of helium was maintained through the chamber to remove any gases that might be released during heating of the cell. When the electrolyte was molten, a sample for analysis was taken by immersing an iron pipe sampling cup into the bath. The electrolyte contained 5.14 percent soluble titanium with an average effective valence (a.e.v.)/ of 2.11. The operating temperature of the cell was 850° C. The cell was now ready for the investigations.
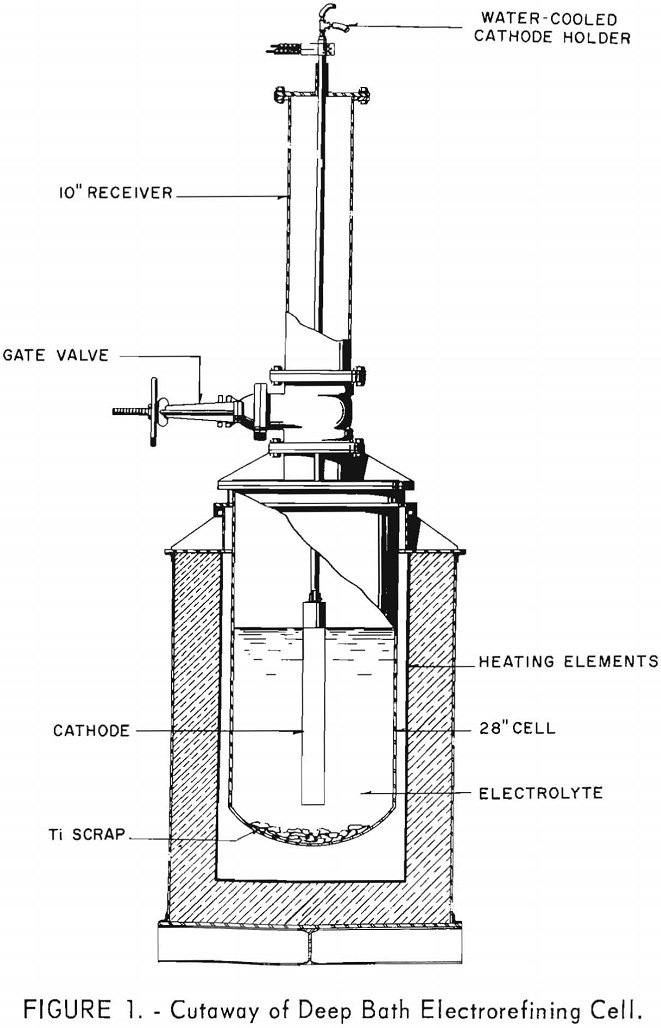
Periodically during these investigations it became necessary to replenish the anode material and mixtures of anhydrous sodium chloride and master mix to maintain the bath level between 39 ½ and 41 inches. This was accomplished by replacing the cathode and water-cooled cathode lead with a specially constructed 8-inch-diameter by 40-inch-long bottom-dumping loader. The loader was so constructed that after an inert atmosphere was reestablished in the receiver chamber and the loader lowered below the 10-inch gate valve, the charge could be dumped into the cell.
Standard Operating Procedure
The cycle of operation, or test run, began with a clean cathode and included the following manipulations:
- The cathode was bolted to the water-cooled copper-cathode lead and sealed in the cell receiver.
- An inert atmosphere of helium was established within the receiver chamber by purging the receiver for 2 hours.
- The 10-inch gate valve, which sealed the receiver from the cell was opened and the cathode lowered until contact was made with the bath, indicated by an ohmmeter.
- The cathode was immersed to a predetermined depth.
- A predetermined quantity of direct current was applied for a specific time to produce a deposit.
- The cathode was withdrawn from the electrolyte until the electrical circuit was interrupted, leaving the cathode and deposit positioned just above the bath, where it drained for 15 minutes.
- The cathode and deposit were raised into the receiver chamber, the 10-inch valve was closed and the deposit was allowed to cool 1½ hours to room temperature.
- The receiver was opened and the deposit was recovered by mechanical stripping.
- The cathode was returned to the receiving chamber to repeat the cycle.
The stripped deposit, which consisted of titanium metal and occluded electrolyte, was weighed, leached with a mechanically agitated solution of water and 5 percent by volume hydrochloric acid in a glass-lined water-cooled tank, washed with demineralized water until free of chloride ion, dried, and sized by screening into +8, -8 +34, -34+48, -48 +120 and -120 mesh fractions. Samples of these sized fractions were evaluated by chemical analysis and hardness determination.
Experimental Data
In general, the investigations were divided into two groups: (1) Determining the feasibility and limitations of deep bath deposition and (2) comparing the deposition on cathodes of various diameters.
The soluble anode consisted of offgrade Kroll sponge placed in the bottom of the cell. The anodic power lead was attached to the cell flange. The sponge had an average Brinell hardness number of 300 and contained the
following percentages of impurities: Fe, 0.40; Mg, 0.12; N2, 0.39; C, 0.15; O2, 0.92.
The first phase of the investigation was performed, using a 36-inch-long, 4-inch-diameter tubular-iron cathode. The scrap titanium in the bottom of the cell limited the average usable depth of the 40-inch-deep bath to 28 inches, leaving a minimum of 6 inches between the bottom of the cathode and the top surface of the anode sponge. This minimum spacing assured that electrical shorting would not occur as the dendritic cathode deposits grew.
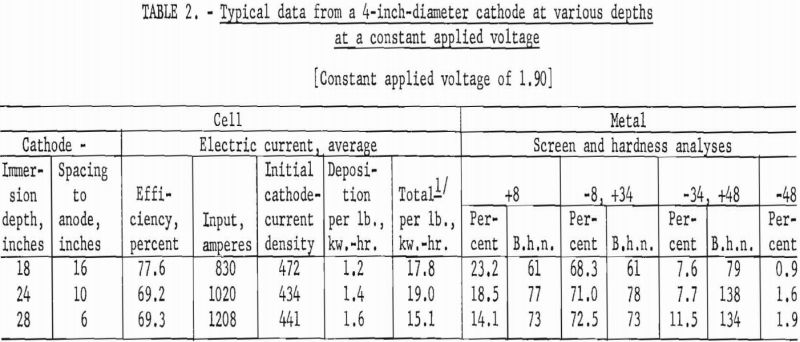
Any differences were ascertained by varying the depths of immersion from 12 inches to 28 at intervals of 12, 18, 24, and 28 inches at constant initial cathode-current densities and then at constant applied cell voltages.
Typical of the numerous cycles of operations performed to determine the conditions are the data – an average of at least eight cycles of operation at each condition – presented in tables 1 and 2.
The major difference brought about by varying the depth of immersion was a change in the deposition kilowatt-hours per pound of metal produced. In general, as the space between the top surface of the anode material and the closest point of the

cathode was decreased, the deposition power per pound of metal produced increased.
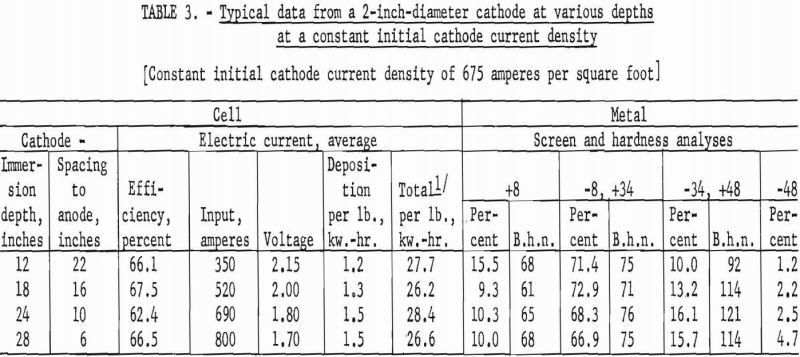
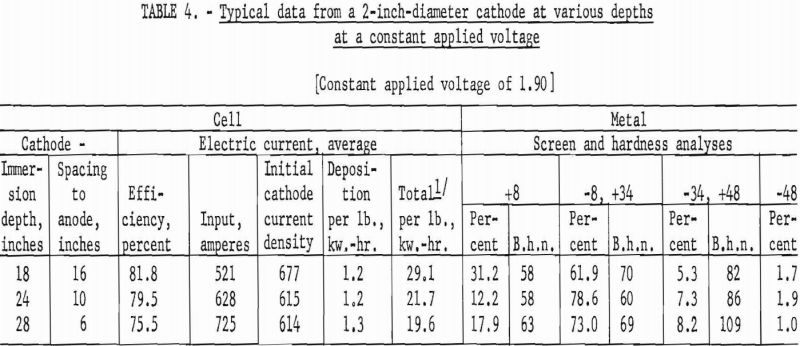
The second series of investigations were conducted, using a 2-inch diameter by 36-inch-long iron tube, under the identical conditions used in the first series. Tables 3 and 4 contain data from representative typical cycles of operations in this series.
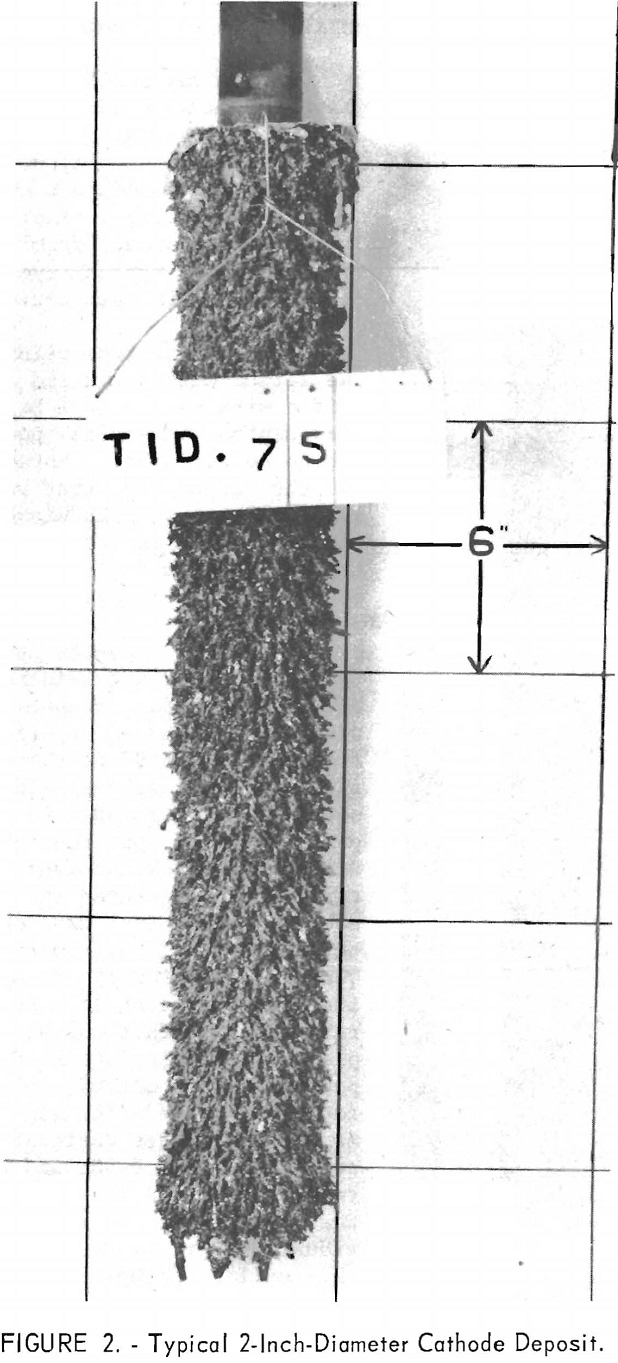
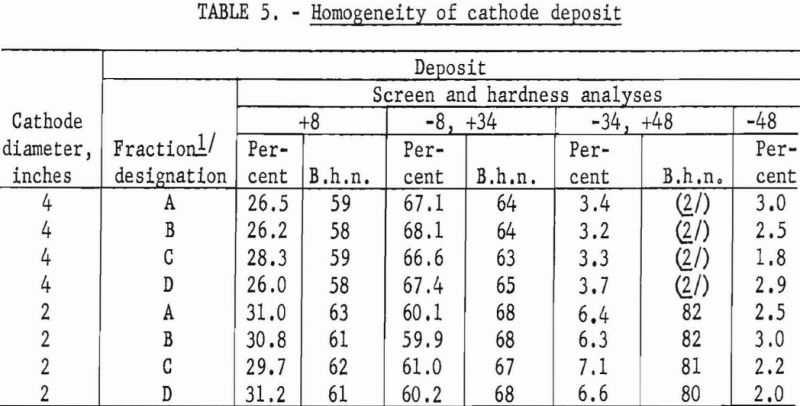
The results from using the 2-inch cathode varied little with change in immersion depths. The total power per pound was greater throughout the series than that required for the 4-inch-diameter cathode, which indicates that the 4-inch cathode is more economical to operate than the 2-inch cathode. Figures 2 and 3 show typical deposits produced on the 2- and 4-inch- diameter cathodes, respectively. Individual deposits from either the 2- or the 4- inch-diameter cathodes did not vary significantly in impurity content, physical condition, or thickness from top to bottom. Presented in table 5 are typical data obtained when a deposit was quartered vertically and each fraction evaluated as a separate entity. This was accomplished by stripping the deposit from the cathode in four equal 6 ½-inch segments, which were designated as fractions A, B, C, and D, respectively. A review of the data shows that an individual deposit produced from this cell on either a 2- or 4- inch-diameter cathode is comparable in quality and quantity from top to bottom.

The second phase of these investigations was to compare the efficiency of various cathodes ranging in diameter from ½ inch to 4 inches. The actual diameters of the test cathodes were 0.53, 1.07, 2.1, and 4.24 inches. The test cycles of operation were performed at initial cathode-current densities of 440 amperes per square foot; the cathode was immersed to leave a 6-inch spacing between the surface of the anodic titanium and the bottom of the cathode.
Typical of the data obtained in these examinations are those presented in table 6.
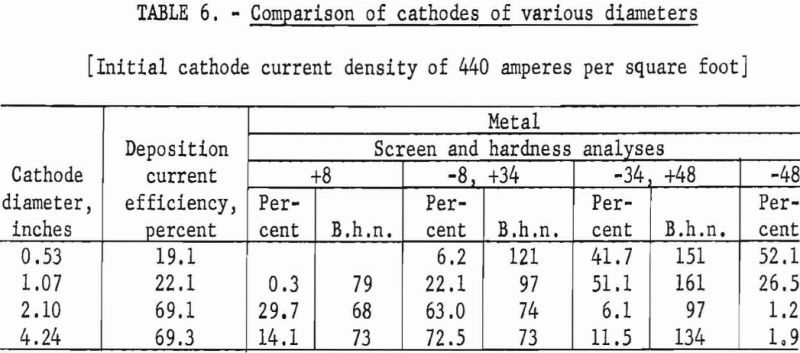
The use of cathode diameters of 1 inch or less was extremely unsatisfactory both for deposition current utilization and metal quality undoubtedly because only very low current inputs could be used to maintain the desired constant initial cathode-current density for comparative purposes.
This investigation was completed by establishing a set of presumably optimum conditions and procedures as shown by previous research, for a long enough time to ascertain the reliability of the assumptions. The conditions were established as follows:
- Cell operating temperature, 850° – 855° C.
- Cell operations performed under a helium blanket at a gage pressure of ¼ pound per square inch.
- Four-inch-diameter iron cathode.
- Twenty-eight-inch immersion equal to 2.56 square feet of surface.
- Initial cathode-current density of 792 amperes per square foot.
- Cell voltage of 3.4 volts.
- Electrolyte contained 4.9 percent soluble titanium, with an average effective valence of 2.18.
- Four-hour deposition time.
The data summarized in table 7 were accumulated from a series of 20 deposits produced under these conditions. In table 8 are data on the metal produced in the 20 tests summarized in table 7.
Conclusion
The efficiency of the deep bath, fused salt, electrorefining cell operation with respect to utilization of d.c. current, improved as the depth and diameter of the cathode increased from 12 to 28 inches and from ½ inch to 4 inches, respectively. Moreover, the metal deposit was distributed evenly on the cathode at all immersion depths, and metal quality was not imparied by deep bath deposition.