In view of the recognition of the potentialities of titanium and its alloys as important structural materials there has arisen a need for a systematic investigation of various titanium binary diagrams. Of these, the Ti-Cr system was of particular interest because of the improved properties imparted to titanium by small chromium additions.
Sponge titanium (99.7 pct) and electrolytic chromium (Bureau of Mines analysis: 99.03 pct Cr, 0.40 pct Fe, 0.53 pct O2) were used for the initial determination of the system. Subsequent refinement of points, particularly for the low chromium side of the diagram, was accomplished by using iodide titanium and high purity chromium (National Research Corp. analysis: 0.050 pct C, 0.045 pct O2).
The Ti-Cr alloys were prepared by melting 25 g charges in an enclosed, water-cooled copper crucible using a movable tungsten electrode. The atmosphere was helium, prepurified by first passing it through
a drying agent and then through sponge titanium heated to 850°C. Particular care was taken to avoid any large scale segregation resulting from insufficient mixing of the components. Each charge was melted and kept molten for approximately 1 min, allowed to cool, inverted, remelted, and kept molten for an additional minute.
The alloys were analyzed for chromium content and although the actual percentage was invariably low the deviation from the nominal composition never exceeded 0.6 pct Cr.
Specimens being prepared for metallographic examination were first subjected to two separate treatments: a homogenizing and a final heat treatment. The homogenizing period consisted of heating the specimens for 4 to 6 hr at 1350 °C followed by water quenching. The final heat treatment consisted of holding specimens for a sufficient time at the temperature to be investigated in order to approach equilibrium conditions.
Evidence obtained during preliminary dilatometer runs indicated a much more rapid phase trans-
formation during heating than during cooling. Subsequent investigation revealed the fact that transformations during cooling were so slow that reliance could be placed only on the heating curves representing the conversion of the low temperature phases to the high temperature phase. This necessitated long-time (two weeks) heat treatments slightly below the eutectoid temperature prior to each run to insure the maximum amount of the low temperature phases.
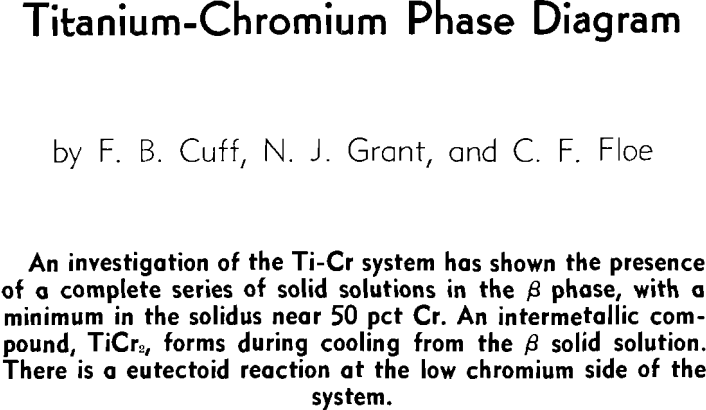
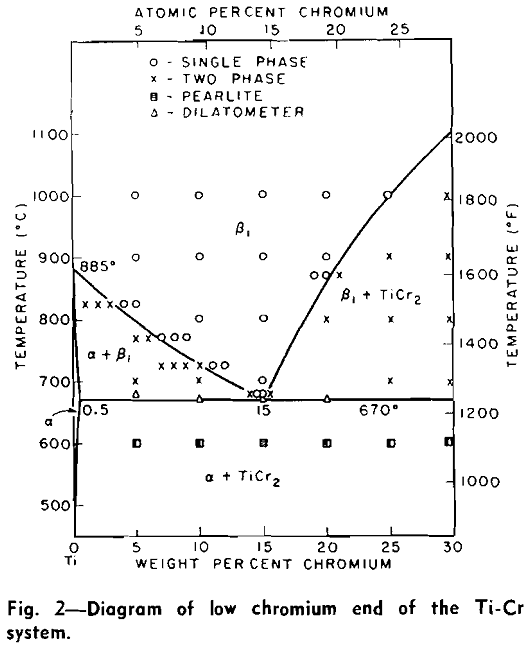
Titanium and chromium form a continuous series of solid solutions which, however, exist only over the narrow temperature range of 1350° to 1400°C for the alloys containing approximately 50 to 70 pct Cr. The minimum in the solidus occurs at approximately 50 pct Cr and 1400°C.
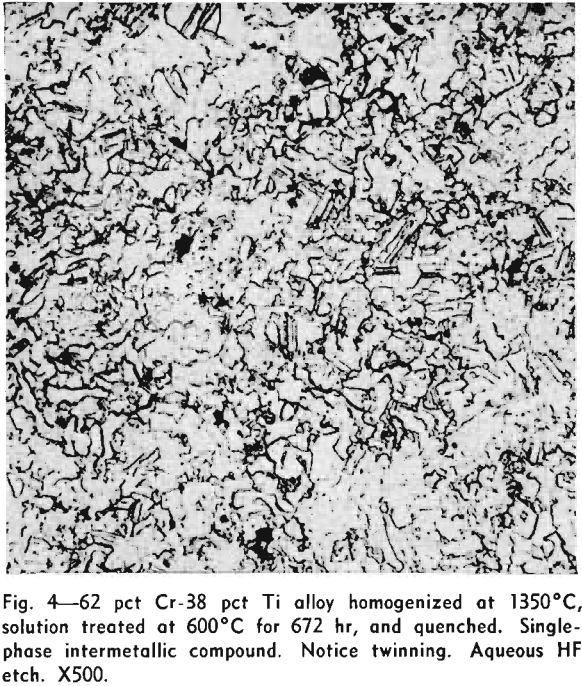
It was found that a compound existed between 60 and 65 pct Cr. This would place it near the stoichiometric composition of Ti2Cr3. However, a Debye-Scherrer diffraction pattern showed the compound to be face-centered cubic with 24 atoms per unit cell and a parameter of 6.91Å. This compound is shown to be TiCr2, which stoichiometrically should contain 68.47 pct Cr. This suggests that the compound, TiCr2, is a defect lattice compound and that the actual position in the diagram does not correspond to the ideal stoichiometric composition.
The dominating influence on the shape of these curves is the total or partial disappearance of the compound (TiCr2) as the eutectoid temperature is reached. Qualitatively, the contraction at this temperature is due to the fact that a large and complex structure (TiCr2) is being replaced by a more compact structure (β). However, the amount of contraction is dependent upon the position of the alloy with respect to the compound.
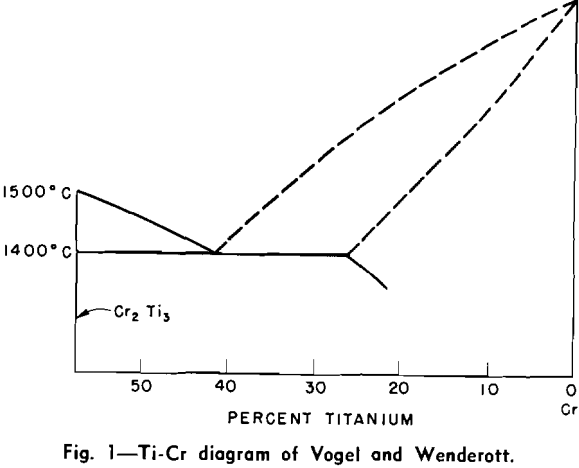
It was found that specimens near the eutectoid composition show a striking Widmanstatten structure when in the as-cast condition. This structure was not observed when the alloys were more than a few percentage points of chromium from the eutectoid composition. This Widmanstatten structure occurs primarily within the grains with litttle or no evidence of it near the grain boundaries.