Unlike base metals which are readily soluble in mineral acids, noble metals like gold and silver require the presence of oxidizing and complexing agents for dissolution. Silver approaches base metal behavior in acidic thiourea solutions (Ag + 3Thio ↔ Ag(Thio)3+ + e, E° = 0.025 V) requiring only mild oxidizing conditions for dissolution. Accordingly, the following reactions characterize the dissolution of silver in acidic thiourea with various oxidants

Ferric sulfate and chloride have been frequently employed as oxidants. Other oxidants that appear compatible with acidic thiourea systems include Na2O2, O3, KBrO3, and K2Cr2O7.
Like the metallic form, certain silver minerals require oxidizing conditions for dissolution in acidic solutions. Argentite (Ag2S) in the presence of thiourea is leached by ferric ions according to the following overall reaction
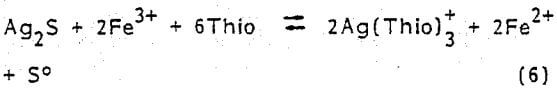
It is reasonable to believe that refractory silver minerals like Hessite (Ag2Te) would respond to acidic thiourea leaching similar to the chemistry represented in Eq. (6).
Cerargyrite (AgCl) is a silver mineral that will dissolve in acidified thiourea solutions under nonoxidizing conditions. This mineral proceeds to solution by simple complexation dissolution. The following reaction represents the dissolution of AgCl in thiourea
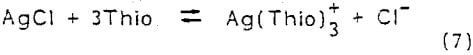
This reaction is favored thermodynamically, at 25°C the equilibrium constant equals 2.2 x 10³. Gubailovskii, et al. (1972 , 1975) studied the kinetics of AgCl dissolution in aqueous solutions of thiourea. Their results suggest that the reaction is controlled by a diffusion mechanism. In acid solutions, thiourea decomposes with parting of elemental sulfur and formation of urea. The dissolution rate is limited by the dense sulfur film that coats the unreacted silver chloride. In a neutral medium (pH 5-7), silver sulfide forms a nonprotective layer on the silver chloride surface. This layer of silver sulfide gradually dissolves in the thiourea.
Early workers such as Plaksin and Kozhukhova (1941) used thiourea to dissolve gold and silver in H2SO4 and H2O2. They observed similar leaching behavior for both metals. Using solutions containing 10 g/l H2SO4 and 0.6 g/l H2O2, they found the dissolution to increase with increasing thiourea concentration in the range of 3-20 g/l (CS(NH2)2]. At these conditions the solutions were clear for up to 30 hr, after which noticeable amounts of elemental sulfur were liberated from the oxidation of thiourea. The addition of FeCl3 effectively stabilized the thiourea for about 72 hr. At H2SO4 concentrations between 5 and 20 g/l, the dissolution was insensitive to sulfuric acid concentration.
More recently Plaksin and Kozhukhova (1960) studied the factors affecting the dissolution of gold and silver in solutions of thiourea. Various oxidizing agents were investigated; however, the rate was found to increase in the presence of Fe3+. The dissolution rate increased linearly with increasing thiourea concentration in the range of 1-90 g/l [CS(NH2)2). Optimum leaching results were achieved with a sulfuric acid concentration between 1 and 5 g/l.
Lodershchikov and co-workers (1975) investigated the kinetics of the dissolution of silver in aqueous solutions of thiourea. Although most of their data support a diffusion mechanism for leaching, some of the results were inconsistent with this interpretation. The complicating role of surface films at some conditions was believed responsible for these inconsistencies it was demonstrated that thiourea is stable in the presence of ferric sulfate. The rate of silver dissolution increased linearly with increasing thiourea concentration until a maximum was reached, at which the rate was insensitive to further additions of thiourea. The maximum impressive results are overshadowed by an extremely high consumption of thiourea. The author concluded that the thiourea technique was uneconomical when compared to traditional cyanidation approaches.
A recent paper by Chen, et al. (1980), discusses the leaching of gold and silver by “acidothioureation.” Figure 4 compares acidic thiourea and cyanide leaching of gold and silver. For the conditions employed and up to 60 min, the rate for the thiourea system is approximately an order of magnitude greater than cyanide for both gold and silver. A copper concentrate containing 1.5 oz/ton (51.4 g/tonne) gold, 7.3 oz/ton (250 g/tonne) silver, and 6.02% Cu was leached with both thiourea and cyanide solutions (same solutions as given in Figure 4, except 0.5% CaO). The recovery of both Au and Ag was slightly higher with thiourea after 6 hr of leaching; interestingly, the level of copper dissolution was significantly lower with the thiourea system.
Ozone has been employed as an oxidant in the acid thiourea leaching of gold (Chtyan, 1976; Chtyan and Babayun, 1977). At 25°C the rate of gold dissolution was highest for a solution containing 61 g/l CS(NH2)2, 0.06 g/l O3, and 10 g/l H2SO4. It was possible to extract about 98% of the gold from a concentrate in 200 min using an ozone-air mixture as an oxidant. In general, successful leaching where aggressive oxidant like hydrogen peroxide and ozone are used requires relatively high concentrations of thiourea. These oxidants consume the thiourea by the rapid oxidation to products where sulfur has a higher oxidation state. In contrast, ferric ion requires lower concentrations of thiourea for optimum leaching.

