Table of Contents
The method usually given for detection of Sulphocyanate (Thiocyanide, Thiocyanate) is to acidify and add a ferric salt, a blood-red color indicating the presence of KCNS. Silver and copper, however, interfere, and if present in sufficient amount may entirely prevent the formation of the characteristic color, so they must be removed before any determination can be made. The silver may be conveniently precipitated by shaking up a portion of the solution in a bottle with a little aluminium dust. There is usually sufficient caustic soda in working solutions to effect this reaction, but if not a tiny splinter of caustic soda may be added. In order to find out when precipitation is complete a little of the solution may be filtered and added to a flask containing dilute sodium sulphide; if any discoloration ensues agitation should be continued, or if necessary more aluminium dust added. If not, the solution may be filtered, and the test proceeded with.
If, after removing the silver, copper is still present, the writer has found that it may be conveniently eliminated by acidifying in presence of an excess of potassium ferrocyanide, the copper being precipitated as insoluble ferrocyanide and the excess of potassium ferrocyanide being removed as prussian blue on the addition of an excess of a ferric salt.
Sulphocyanate/Thiocyanide/Thiocyanate Assay Procedure
If neither silver nor copper exist in the solution, or if silver alone was present and has been thrown out as described, take 100 cc of solution, acidify with a measured quantity of sulphuric acid, say 10 cc, add 10 cc of solution of a ferric salt (preferably ferric sulphate), and titrate with decinormal permanganate until the red color of the ferric sulphocyanate is dispelled.
If ferrocyanide is present it will come down as prussian blue, and must be filtered out, and it is safer to filter always, Since small amounts of prussian blue may not be detected from the appearance of the liquor, and yet may be present in sufficient amount to impair the accuracy of the subsequent titration. When much prussian blue is produced, to avoid washing the precipitate, take an aliquot portion of the filtrate for titration, say 60 cc which in this case represents 50 cc of the original solution to be tested.
If the amount of ferrocyanide is large it may be necessary to add more ferric salt, because the red ferric sulphocyanate will not be formed until the whole of the ferrocyanide has been changed into prussian blue.
When Copper is present in the solution to be tested (silver being absent, or having been precipitated and filtered out as described), take 100 cc, add, for example, 5 cc of 1% solution of ferrocyanide, then 10 cc of sulphuric acid, and then 5 cc of ferric sulphate, making 120 cc in all. Filter, and measure out for titration 60 cc of the filtrate, representing 50 cc of the original solution, and titrate with N/10 permanganate.
The preparation of the decinormal permanganate has been already described. (Ferrocyanide determination method.)
1 cc N/10 permanganate = 0.001619 gm. KCNS.
Sharwood considers the determination by permanganate unreliable and prefers to estimate sulphocyanate colorimetrically, using the reverse process to that described for small amounts of iron. The writer, however, has found the permanganate method to give closely concordant results when tested on made-up solutions, provided that silver and copper are first eliminated.
Potassium Sulphocyanate Method for Estimating the Iron
If the quantity of iron is very small it is best estimated colorimetrically by at once dissolving the precipitate in hydrochloric acid and carefully washing the filter paper. A standard solution of ferric iron is prepared by dissolving 0.1 gram of C.P. iron wire in nitrohydrochloric acid. Dilute the solution thus obtained and precipitate the iron with ammonia. Filter out the ferric hydroxide and wash thoroughly and then re-dissolve in dilute hydrochloric acid and add distilled water to make up to 1 litre. The test is made similarly to that described for the colorimetric estimation of copper. Two colorless glass cylinders marked at 100 cc are taken. In one of them is placed 1 or 2 cc of hydrochloric acid and 10 or 15 cc of a solution of potassium or sodium sulphocyanate of such a strength as to have an excess of the latter after running in the standard iron. Fill up nearly to the mark with distilled water leaving space for addition of the standard iron. Into the other cylinder pour a similar quantity of hydrochloric acid and sulphocyanate solution and a measured amount of the solution to be tested filling up to the mark with distilled water. The formation of a blood-red color will indicate the presence of sulphocyanate. Standard iron solution is then added from a burette to the first cylinder until the color corresponds with that of the solution being tested. Should the first cylinder lack much of being filled to the mark by this time a little more water may be added and if necessary a few more drops of the iron solution. Here as in the copper estimation it is well to enclose the cylinders in a box open at the bottom in such a way that the light may be reflected upward from a sheet of white paper and the color compared by looking down vertically through the column of liquid. Care should be taken not to have so much iron present in the test as to make the color too deep for comparison. When adding standard solution from the burette it is important that the contents of the cylinder be well mixed after each addition, and for this purpose it is better to turn the liquid out into a beaker each time.
Reduction Method for Estimating the Iron
Should the amount of iron obtained from the cyanide solutions be large enough to be determined by reduction and direct titration, the procedure may be as follows: onto the filter paper containing the washed precipitate of ferric hydroxide pour some dilute sulphuric acid to dissolve it, taking care to wash the paper free from iron. Clennell recommends boiling this solution with clean aluminium turnings until a drop of the liquid no longer imparts a red tint to a drop of potassium sulphocyanate solution. The flask is then cooled rapidly without removing the aluminium and titrated with standard potassium permanganate. Evolution of hydrogen must have entirely ceased before titration.
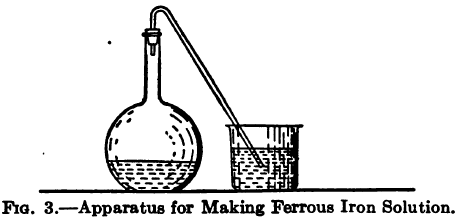
The decinormal permanganate solution is made by dissolving 3.156 grams of the pure dry salt in distilled water and making up to 1 litre.
1 cc of this solution = 0.0056 gram Fe or 0.0369 gram K4Fe (CN)6 (potassium ferrocyanide).
If the permanganate is pure and dry the solution made up as above will be almost exactly decinormal, but it may be standardized against an iron solution, if desired.
Fit a boiling flask with a rubber stopper through which passes a short length of glass tube to act as a steam vent, or better still, a bent tube long enough for its free end to pass below the surface of some water in a beaker. Remove the stopper and pour in about 100 cc of dilute sulphuric acid and a few grams of sodium carbonate to expel the air by formation of carbonic acid. Then place in the flask 0.1 gram of pure soft iron wire previously cleaned with scouring paper, and allow to dissolve. 0.1 gram of such iron wire may be considered to contain 0.0996 gram of pure iron. Cool the solution rapidly and titrate with the permanganate solution until a faint rose color remains permanent. With a strictly decinormal solution of permanganate 17.85 cc should be required to oxidize 0.1 gram of iron, or 17.78 cc for the 0.1 gram of wire taken.
Ferrocyanide Determination Method
The most reliable assay method is probably that of determining the iron and calculating to ferrocyanide.
It must of course be assumed that all the iron found occurs in that form, but as ferrocyanides are most unlikely to be present in ordinary circumstances the assumption is a fairly safe one.
As in the case of the copper determination especial care must be taken to decompose the cyanogen compounds, either by evaporating with nitric and sulphuric acids till white fumes are given off or by evaporating twice with nitric acid to dryness and then with strong sulphuric to white fumes; and as before, if dealing with a large volume of original solution it is better to begin by removing the lime with sodium carbonate or potassium oxalate. The solution resulting from the evaporation is cooled, diluted and boiled to dissolve anhydrous salts. It is then cooled again, neutralized with ammonia in slight excess, and heated to boiling. The ferric hydroxide formed is filtered out and washed on the filter.
