The test outlined is a simple and rapid method for the determination of total cyanide in plant solutions, providing significant amounts of iron cyanides are not present.
Copper, nickel, zinc, and iron cyanides build up in plant cyanide solutions as a result of the usual gold milling practice of recycling solutions . Since these metals are present in the plant solutions as cyanide complexes, a determination of the total cyanide concentration for comparison with the free cyanide concentration is useful as an indication of how the sodium cyanide added is being consumed, and of the approximate concentration of metals present in the solutions.
The methods for total cyanide used in the past involved either caustic addition or distillation. The caustic addition method determines only the “free” cyanide plus the complex cyanides of zinc. The distillation technique gives the total cyanide present but the method is time-consuming and requires extra equipment that may not be always convenient to use in the mill laboratory. The technique described here involves only a simple addition of the reagent diethylenetriamine, (DETA) followed by a silver nitrate titration to determine “free” cyanide. However, the cyanide present as ferro-or ferricyanide is not measured and therefore will not be included in the total cyanide indicated.
PROCEDURE
A 2 ml portion of 10% diethylenetriamine in water is added to the 25 ml sample of cyanide solution under test and the mixture is then titrated for cyanide content in the usual manner with standardized silver nitrate, using potassium iodide as the indicator. The end-point taken is the first faint permanent bluish opalesence. It should be noted that the blue color appears first and the opalesence which indicates the end point occurs with further silver nitrate addition.
The “free” cyanide value must be subtracted from this titration result to obtain the complexed cyanide concentration.
RESULTS
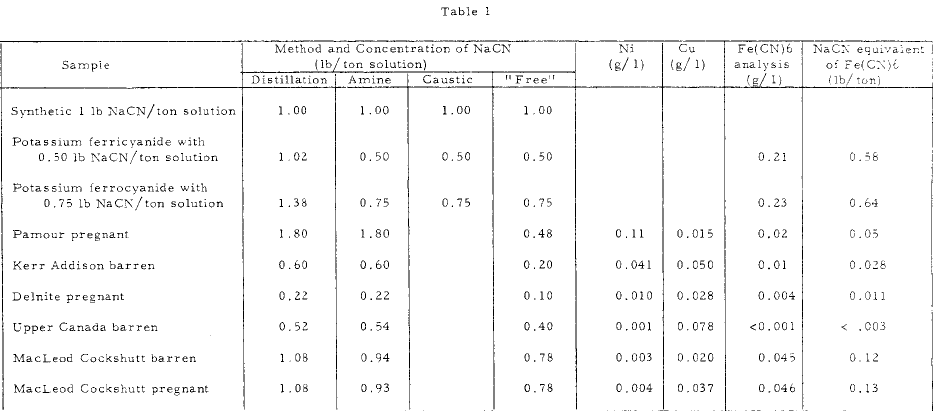
A few of the results obtained in the Mines Branch laboratories using the various methods for determining complexed cyanide compounds are shown in Table 1 .
DISCUSSION
The results show that the procedure using DETA results in a total cyanide value which is in close agreement with the distillation technique, providing significant amounts of the Fe(CN)6 complexion are not present. In tests on solution where Fe(CN)6 complex ion was present, as in the MacLeod Cockshutt solution, and in the synthetic solutions, the sodium cyanide equivalent of the Fe{CN)6 content was approximately equal to the difference between the total sodium cyanide by the distillation procedure, and the sodium cyanide by the DETA. procedure . Thus it appears that the DETA procedure indicates the amount of cyanide complexed with other metals; mainly zinc, copper and nickel. Since the zinc complex present in gold plant solutions is usually low and reasonably constant, variations in the total cyanide by the DETA method indicates variation in the copper and nickel concentrations in the solutions. The presence of these metals is also indicated by the bluish colour which develops in the titration.
Furthermore, the use of the DETA method in conjunction with (a) the usual free cyanide method, (b) the method using a caustic addition prior to the AgNO3 titration, and (c) the distillation technique, will give the mill operator an approximation of the relative contents of the cyanide complexes of zinc, iron, and nickel and copper in the plant solutions . The distillation technique may be avoided if the iron content is determined by normal methods, and the iron cyanide complex content calculated from the values obtained.
THE GOLD-LEAF TEST METHOD
Apparatus
Eberbach variable speed clinical shaker,
Canadian Laboratory Supplies, Toronto,
Catalogue No. 100, Item No. 19-970
NOTE: This unit comes with a pipette holder which must be replaced with a simple test-tube adapter.
-16 x 150 mm test-tubes fitted with No . 6 cork stopper (not rubber stopper)
-23 K gold – leaf booklet, Central Scientific Company, Toronto, Catalogue JC 154, No. 88345
Procedure
The gold-leaf test is a method of evaluating the dissolving power of cyanide solutions by comparing the dissolution times in such solutions of uniform amounts of standard gold-leaf.
The gold-leaf is best removed from the booklet by clipping together with small paper clips, an overlay of metric graph paper and the tissue paper under the leaf, and then tearing the tissue paper at the fold. The gold-leaf between the two clipped sheets can then be cut along the appropriate lines of the graph paper to give a square of one centimeter to the side. The gold-leaf is placed on the end of the cork stopper by first moistening the end of the stopper by touching it to the tongue and then contacting the moistened tip with the cut square of leaf. If the graph paper adheres to the leaf, it is easily removed by pressing a corner slightly with the fingernail.
A 10 ml portion of the solution to be tested is placed in a test tube, and the cork stopper, with the gold-leaf adhering, is inserted. Momentary vigorous shaking is usually necessary to free the gold-leaf from the stopper. The test tube is then shaken in a horizontal plane on the mechanical shaker at a rate of approximately five oscillations per second. The amplitude of the stroke is fixed at about 1 inch. The dissolution of the gold can be followed visually without difficulty, provided the tubes are under a good light. The solutions are compared on the basis of the length of time required for complete dissolution of the gold-leaf in each solution. The test is repeated from 3 to 5 times and the average value taken.
The stoppers are used only once and then discarded. To obtain reproducible results the surface of the cork stopper which is in contact with the solutions should be comparatively free of defects. It is not necessary to use the shaker described under Apparatus . Some mills are using their Ro-tap screen shaker adapted to hold test tubes . At the Mines Branch, a laboratory Wilfley Table mechanism is used.
Comments
Experience has shown that the length of time required for complete dissolution of the gold varies depending on the type of cyanidation circuit in use. Thus the solution for mills where the whole ore is cyanided will normally dissolve the gold-leaf in from 0,5 to 1.5 hr, while solutions from mills where a concentrate is cyanided will require from 1 hr up to as long as 12-15 hr.
Pure cyanide solutions containing approximately 1 lb/ton sodium cyanide and 1 lb/ ton lime will dissolve the gold-leaf in 10-15 min.
The significance of the results of the gold-leaf test is dependent on the mill circuit. For example, an indicated dissolution time of 8 to 10 hrs might be acceptable if the total contact time in the mill circuit was 72 hrs, while the same dissolution time might have serious implications if the mill contact time was only 24 hrs .
The test may be used to control the discarding of barren mill solution, which is usually necessary to prevent the build-up of impurities in the circuit. Thus the quantity of barren solution discarded could be regulated so as to hold the gold-leaf dissolution time to a predetermined figure known from experience to be permissible for a given mill. In addition, where the solutions in a gold mill have become fouled, the test may be used to determine if the fouling gradually increases throughout the mill circuit, or if it sharply increases at some particular point.
