Table of Contents
Zeolites were first recognized as a new group of minerals by Cronstedt with the discovery of stilbite in 1756. The word zeolite was coined from the two Greek words meaning “to boil” and “a stone” because of the loss of water when heated in the mineralogist’s blow pipe.
In 1845 it was discovered that certain soils have the power of retaining ammonium salts. These were the first ion exchange experiments. Later it was discovered that it was the hydrated silicates in the soil that produced this phenomenon and several years later a paper was published dealing with the action of dilute salt solutions on silicates which showed that this base exchange principal, which we now call cation exchange, is reversible. The quantitative cation, exchange behavior of zeolite minerals, such as chabazite, was studied.
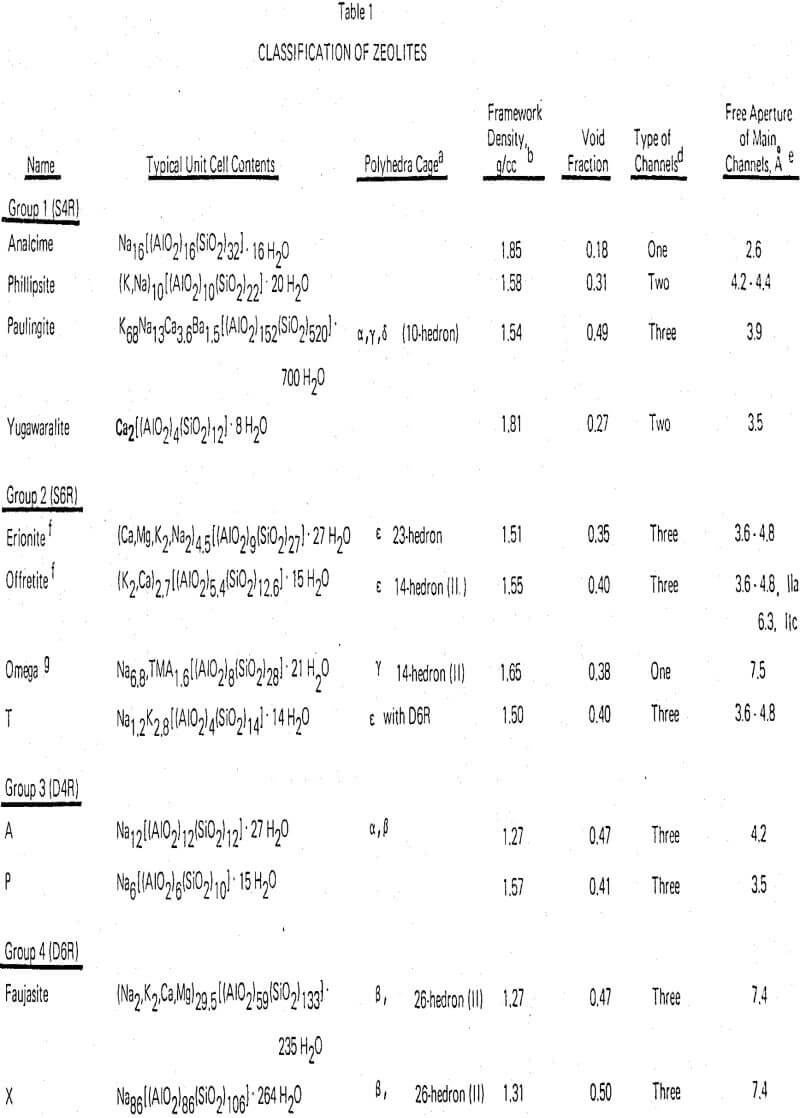
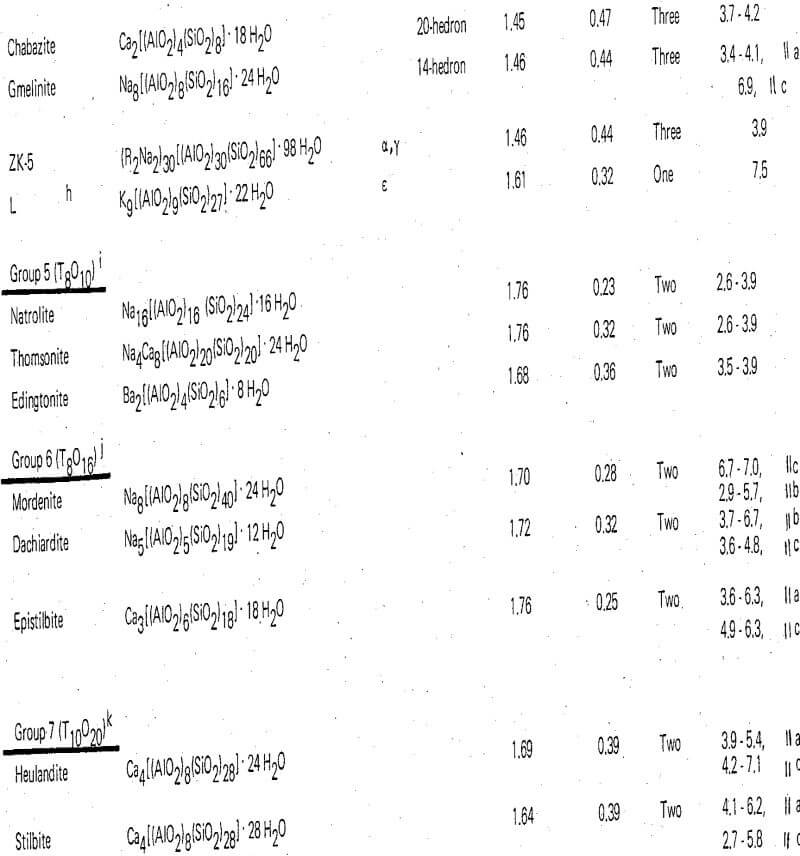
Zeolite Structural Chemistry
Along with the feldspars the zeolites are framework silicates; their structures consist of a three-dimensional framework of SiO4 and AlO4 tetrahedra. As in the feldspars the trivalent aluminum in these tetrahedral positions requires the presence of additional positive charge such as alkali metal or alkaline earth ions in order to maintain electrical neutrality. In the zeolites, for stability reasons the maximum substitution of aluminum for silicon that occurs is in the ratio of 1:1. In this event a complete ordering of the aluminum, and silicon is generally present. The minimum substitution, that is, the zeolite with the maximum Si/Al ratio, is probably mordenite with a ratio of 5. However, unlike the feldspars which have tight and dense structures, zeolite structures are open and contain large cavities filled with water molecules. The cavities may be interconnected in one, two, or three dimensions producing, upon dehydration, a crystal permeated with channel systems in one, two, or three dimensions. The metal ions which are needed for the charge compensation occupy sites in the channel or adjacent to the cavities and are generally available for exchange by other cations.
In many cases the zeolite framework structures consist of simple polyhedra, each polyhedron in itself is a three-dimensional array of the individual tetrahedra in a simple geometric form. These arrangements of polyhedra are referred to as secondary building units.
Eight of the twelve sodium ions lie in the center or near the center of the six rings in the hexagonal faces and four occupy positions adjacent to the larger eight membered ring. Dehydration of the zeolite leaves these four cations in position snear the eight-membered ring where they protrude into the apertures and may interfere with the movement of molecules. When replaced by other cations, such as calcium, the adsorption behavior changes. Since one calcium ion replaces two sodium ions, the cations are removed from the eight-membered rings and the zeolite has a larger effective pore size. Replacement of the sodium by larger univalent ions, such as potassium, restricts the eight-membered ring even more and produces a zeolite of a smaller pore size.
Some Aspects of Zeolite Chemistry
Zeolites useful as molecular sieve adsorbents and catalysts do not exhibit any change in structure when they are dehydrated under very severe conditions. Although in many zeolites water molecules affect the locations of the exchange cations, they do not have a primary structural function. In the stable zeolites the desorption of water is exhibited as a continuous plot when shown as a thermogravimetric curve of loss in weight vs. temperature. When hydrated the zeolite is a polyanionic framework, which surrounds a water solution of positive ions. After dehydration the stable zeolite can withstand a temperature of about 700°C.
In the laboratory, zeolites are dehydrated before use by heating to a temperature of at least 350°C in a vacuum, in commercial practice dehydration is brought about using a dry purge gas such as air, nitrogen, or methane in order to reduce the partial pressure of water vapor in contact with the zeolite.
It is likely that hydroxyl groups exist on the terminal surfaces of the zeolite crystals but little is known about the terminal surfaces. Investigation of the formation and nature of hydroxyl groups in zeolites has been extensive and has utilized various techniques including thermal analysis and infrared spectroscopy. Hydroxyl groups are formed by two methods:
- Deammoniation of NH4+ exchanged zeolites.
- Hydrolysis of polyvalent cations. These methods are illustrated in Figure 5.
Synthesis of Zeolites
Extensive studies have been carried out,in an attempt to synthesize “minerals.” In the preparation of zeolites, early investigators attempted to duplicate what was then supposed to be a natural process without too much success. In the late 1940s, the initial attempts carried out by Union Carbide scientists were based on a new departure and a low temperature process using reactive ingredients. These initial results were very successful and resulted in the preparation of many different types of synthetic zeolites.
This approach was primarily based upon the use of a freshly prepared, highly reactive aluminosilicate gel or hydrogel. The term “gel” in this sense means a hydrous metal aluminosilicate mixture which is prepared either from aqueous solutions of reactive solid phases. Typical gels are prepared from aqueous solutions of sodium aluminate, sodium silicate, and sodium hydroxide, or perhaps a different alkali metal such as potassium. The zeolites are formed when the gels are crystallized at temperatures ranging from room temperature to 200 °c at atmospheric or autogenous pressure. This method is well suited for the alkali metals since they form soluble hydroxides, aluminates, and silicates and it is possible to prepare reactive and homogeneous gel mixtures.
The gel structure is produced by the polymerization of the aluminate and the silicate anions. The composition and structure of this hydrous polymer gel appears to be controlled by the size and structure of the polymerizing species. Differences in the chemical structure and molecular weight distribution of the starting species in the silicate solutions lead to differences in the gel structures and major differences in the zeolite phases produced. During the crystallization of the gel, the sodium ions, aluminate, and silicate components undergo a rearrangement into the zeolite crystalline structure.
Application of Synthetic Zeolites as Molecular Sieves
The two major applications of synthetic zeolites are adsorption arid catalysis, Zeolites are high-capacity selective adsorbents for two reasons:
- They separate molecules based upon molecular size and configuration, of the molecule relative to the geometry of the zeolite structure.
- They adsorb molecules, in particular those with a permanent dipole moment and other interaction effects, with a selectivity that is not found in conventional adsorbents.
Two types of separation may occur. One based upon the molecular sieve effect and the second upon preferential adsorption. The sodium form of zeolite A will readily adsorb materials such as water, carbon dioxide, sulfur dioxide, and all hydrocarbons containing one or two carbon atoms. Propane and higher hydrocarbons are physically excluded with the exception of propylene which is adsorbed slowly. When the sodium ions are exchanged by calcium the effective pore size increases at a point corresponding to about 30% exchange by Ca++.
If two molecules are both capable of entering the micropore system, then the zeolite retains one preferentially to the other on the basis of polarity or other molecule-zeolite interaction effects. Polar and unsaturated molecules are selectively adsorbed. Water, a strongly polar molecule, is very selectively adsorbed. The zeolite cavities are essentially filled with water at very low concentrations and, in the case of zeolite A each cavity retains 20 molecules at vapor pressures as low as 1/1000 of an atmosphere at room temperature. The cavity is essentially filled. In the case of gases such as nitrogen and oxygen, nitrogen is preferentially adsorbed due to the interaction of the quadrupole of the nitrogen molecule with the cations of the zeolite.