Definition of Solvent Extraction: The unit operation “solvent extraction” is applied in many industries, and has several different forms.
For instance, for the recovery of many vegetable oils from naturally occurring products, a process is adopted in which the raw material is washed in several counter-current stages by a solvent which removes the required oil, an example of this being the extraction of soybean oil using trichloroethylene. In this form, solvent extraction is really a leaching operation.
In the petrochemical industry, aliphatic and aromatic compounds are separated by solvent extraction which in this case means the countercurrent washing of the mixture with a solvent into which one component of the mixture is extracted. Use is made of the distribution coefficient of the solute being extracted between its original feed solvent and the extracting solvent.
In both the above operations, the “extraction” is achieved because of the physical properties of the systems involved, the solubility of the solute in the solvents being of prime importance.
It must be understood that solvent extraction as applied to the recovery of metals is a fundamentally different operation from those mentioned earlier in that chemical reaction plays a major part in the operation. A more correct term for the operation would be “liquid ion exchange”.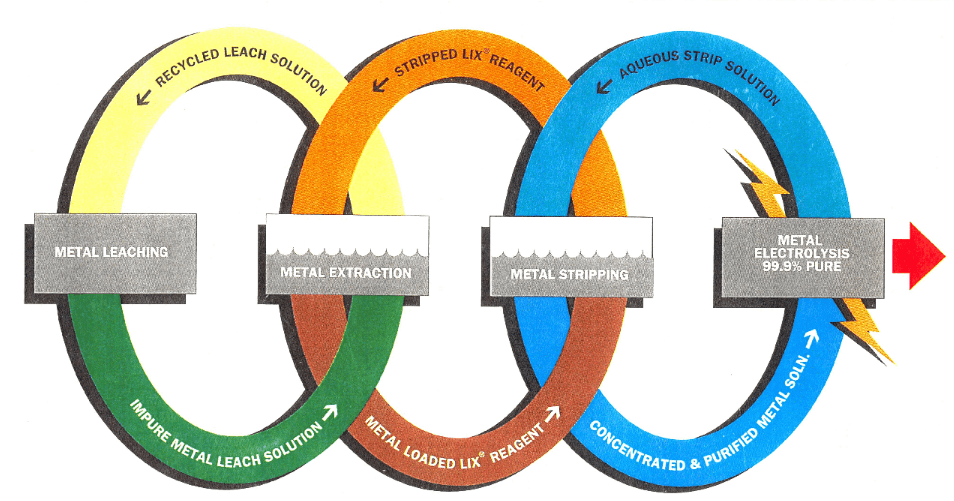
Solvent Extraction applied to Metal Recovery
Having redefined solvent extraction in application to metal recovery, let us now examine how the process works.
The fundamental objective of the operation is to remove a particular metal ion or group of ions from an aqueous solution, leaving behind those ions which are not required. The extracted ion must then be recovered in a useful form.
These objectives are achieved by contacting the aqueous solution with an extracting solvent made up of an active organic reagent dissolved in an organic carrier.
Taking the general case of an aqueous solution of a metal salt of a mineral acid (MA) being contacted with an organic solution of a reagent (RH), a reaction occurs as follows :
![]()
The organometallic compound (RM) dissolves in the carrier and the required metal value M+ is thus separated from the aqueous system.
The production of an organic solution of an organo-metallic compound is of no great value in itself since we require the metal in a usable form. This can be achieved in a variety of ways depending on the metal and the type of organic reagent. For instance, in copper extraction, the loaded organic solution is stripped with a high strength sulphuric acid solution to produce a strong solution of copper sulphate suitable for electrolysis.
In several cases the production of a pure aqueous solution of a metal salt is the final objective and the solution is re-used in processes such as plating or the salt is recovered by crystallisation.
We shall also be discussing techniques in which the loaded organic solution is treated by a reducing gas to produce metal directly.
Which Metals can be Treated by Solvent Extraction
The major applications of solvent extraction in the metallurgical industries stem mainly from the work carried out during the period 1945-55 by the U.S.A.E.C and the U.K.A.E.A. for their atomic energy programmes. Solvents and processing techniques were developed for the extraction and separation of uranium and thorium and the recovery of metals from spent nuclear fuels.
Since then similar work has been carried out on a very wide range of metals and solvent extraction is now regarded as a major route to the production of the rare earths and for the separation of hafnium and zirconium. But these uses, though interesting from a scientific point of view, are of no great interest to Power-Gas because metal outputs are low and plants are very small in size and capital cost.
The metals falling in the above category include:
Thorium, Rare earths, Yttrium, Scandium, Niobium, Tantalum,
Vanadium, Beryllium, Zirconium, Hafnium, Molybdenum, Tungsten.
The major interest for Power-Gas lies in the application of the technique to the recovery of the major non-ferrous metals such as copper, nickel, zinc, and cobalt. These metals have been considered most deeply in Power-Gas R and D work in this area.
- Basic Process Reaction and Design Parameters
- High Selectivity of LIX 64n for Copper
- Stripping Section — Two-Stage Mixing-Settling
- Leach Liquor Throughput Determines SX Solvent Extraction Capital Cost
- Why Mixer-Settler Units are Preferred Contactors
- Choosing the Number of Extraction Stages
- Maximum Stripping Efficiency is Essential
- How to Minimize Losses of Organic Solvent
- Coalescence Beds versus Flotation Units
- Acid Consumption & Overall Operating Costs
Copper Recovery by Solvent Extraction
A common method of copper recovery from oxide ores is dump leaching in which the excavated ore is crushed and dumped on an impervious surface in “lifts” of anything from 10 to 100 ft deep. Sulphuric acid is sprayed onto the levelled off dump via a network of pipes designed to give even distribution. The leach solution so produced has a pH of about 2, a copper content, of the order of 1-2 gm/litre and is usually contaminated by ferrous and ferric iron along with small concentrations of a large number of other metals.
In the past it has been the practice to recover the copper from this type of operation by a process known as “cementation”. This rather crude operation consists of running the solution into launders in which is dumped scrap iron.
A displacement reaction takes place in which copper is deposited whilst the iron in the scrap replaces it in solution, thus producing an impure copper and a leach solution with an increased iron content. The copper has to be sent to a smelter to be refined and although the leach solution can be recycled to the leach operation, the increased iron content leads to the formation of iron caps within the dump thus affecting the efficiency of leaching.
With sulphide ores of copper, a similar type of leach solution can be produced by bacteriological leaching and although this is not practised widely as yet, the results of natural bacteriological leaching are seen in many sulphide copper ore mine waters.
With the object of finding a better method of treatment for these leach liquors, General Mills Inc. of the U.S.A., began an investigation of suitable extraction reagents for copper. In the early 1960’s they developed a solvent, designated LIX-63 which was suitable for the extraction of copper from solutions with a pH above 4.0. This was satisfactory for treating ammoniacal leach solutions but did not solve the more general acid leach problem. In 1965, however, they announced a new reagent LIX-64 , which was designed to operate in solutions of pH 2.0. Largely due to the conservatism of the industry, the new technique did not find immediate application. Fortunately, at about that time, a small Arizonan mining company, Ranchers Exploration, took a lease on an oxide deposit which no-one else wanted because the copper content was only 0.5%, and decided that solvent extraction was the only route to a viable operation.
The operation was set up and the plant at Bluebird Mine became the World’s first copper solvent extraction plant, producing about 6000 short Tons/year of cathode copper from a leach liquor containing 2.8 gm/litre copper. Since then the Bagdad Copper Company, also in Arizona has set up a plant producing 8000 short Tons or copper/year from a more dilute liquor and N.C.C.M. in Zambia have commissioned P.G.C. to design a plant to produce 60,000 Tons of copper/year, the largest solvent extraction plant in the World.
The extraction of copper from aqueous solution by LIX-64N (the successor to LIX-64) proceeds according to the following equation.
![]()
It will be seen that the extraction of copper into the organic phase is accompanied by an increase in the acid level of the aqueous phase. This fact must be borne in mind when considering the type of liquor which can be treated in this way.
It may be said that LIX-64N is a very effective extractant for copper from solutions with a pH in the range 1.5 to 2.8. Below a pH of 1.5 the loading capacity falls off quite sharply and at a pH of 0.5 it is about one-fifth of that at 1.5. Provided the initial pH of the aqueous solution is sufficiently high it is possible to achieve high percentage extractions even for copper contents as high as 30 gpl, although in general the limit for practical purposes is about 3.5 gpl.
The selectivity of LIX-64N for copper is very high in comparison that that for the metals with which it is commonly associated. The only metallic ion picked up to a measurable extent is ferric iron. Even so, the ratio of copper to ferric iron extracted is of the order of several hundreds to one, the exact figure depending on the conditions prevailing for the particular leach solution. Once picked up the iron remains in the organic solution until the stripping stage of the process where it is removed with the copper into the strip liquor. In the case of this operation this is spent electrolyte from copper electrowinning which recycles through the stripping section picking up copper before returning as electrowinning feed liquor.
It is preferable to limit the ferric iron content of the electrolyte feed to an electrowinning process to about 2 gpl as above this figure corrosion of the cathode suspension loops becomes more troublesome and the current efficiency begins to fall appreciably. Because all the iron picked up in the extraction stage is stripped from the organic phase by the spent electrolyte it is necessary to take a bleed from the electrolyte to maintain the iron content of the electrolyte at the preferred level. Some of the iron is removed from the electrowinning circuit by drag-out with the cathodes and the net effect is that the bleed flowrate required is usually less than 1% of the initial leach liquor flowrate. For many applications, the bleed passes back into the leaching operation so that only a very small fraction of its copper and acid content is lost.
So that a clearer picture can be given of the critical parameters which need to be examined in the economic design of these plants, there follows a description of a flowsheet (Fig.1) for the application of solvent extraction to a typical leach situation.
The leach liquor is pumped from the leaching circuit through polishing filters into the extraction section of the solvent extraction plant. The filters may or may not be necessary according to the quality of the liquor leaving the leach plant, but in view of the problems which can arise due to solids build-up in the solvent extraction plant, it is important to consider at an early stage in the design the possible filtering requirements for each particular plant location.
The extraction section of the solvent extraction plant consists of a number of mixer-settlers in series. The aqueous leach liquor flows through these counter-current to an organic stream which is made up of LIX-64N dissolved in a kerosene carrier, and the copper content of the leach liquor is transferred into the organic phase. In order to provide the necessary head to drive the two solutions through the mixer-settlers, the mixing impellers are designed with pumping characteristics. Some previous designs have attempted to separate these duties by providing pumps to transfer the mixed phases into the settler. The high shear imparted by the pumps seriously increased the degree of secondary haze in the separated phases after the settler, giving rise to much increased loss of the dispersed phase.
It is usual to operate the extraction mixer-settlers with an organic/aqueous ratio of 1:1. At higher ratios, lower concentrations of extractant could be used but the overall solvent inventory of the plant would be increased, thus increasing the size of most of the equipment. At lower ratios, the higher organic extractant concentrations which would be required would aggravate iron pick-up problems. Also, it would be more difficult to operate with the aqueous phase dispersed.
It is preferable to operate the final stage extraction mixer-settler with an organic continuous dispersion so that losses of organic material are reduced to a minimum. There is, however, always some dispersed organic material in the aqueous raffinate.
The aqueous raffinate leaving the final stage mixer-settler is pumped into a hold-up tank, where any accidental massive carryover of organic material can be caught. The raffinate then flows to the solvent recovery plant where, depending on the conditions at the particular site, flotation units or coalescing beds separate the greater part of the dispersed organic material and return it to the extraction circuit. The aqueous stream is then returned to the leach operation.
The organic solution, loaded with copper, leaves the first stage extraction settler and is pumped to the stripping section of the plant. This usually consists of two mixer-settlers through which the loaded organic solution and the aqueous electrolyte flow countercurrently. The electrolyte has a free H2SO4 content in the range 150-200 gpl and is loaded to about 40-50 gpl of copper in the stripping plant.
Depending on the preferred method of operation of the electrowinning plant the copper drop can vary from 2 gpl to 20 or even 30 gpl. If a very low copper drop is chosen, the flow of electrolyte is high and the organic/aqueous overall flow ratio in the stripping section is low, with the result that the solvent extraction equipment is larger. In general this method of operation is not preferred, even though very stable operation of the mixer-settlers is possible with high aqueous throughput, because high flows can produce high organic carryover to the cells impairing cathode quality. The usual copper drop is of the order of about 10 gpl, operating in the range 30-40 gpl. This gives an organic/aqueous flow ratio of about 5:1. It is usual to operate the first stripping mixer-settler with an organic continuous dispersion to reduce carryover of organic material into the aqueous phase. Overall ratios greater than 5:1 are required if higher copper drops are used and these conditions can be achieved by including an aqueous recycle stream which stabilises mixer-settler operation whilst maintaining overall stripping characteristics.
The organic stream, stripped of the major part of the copper, leaves the second stage stripping mixer-settler and passes back to the extraction section. The aqueous, loaded electrolyte leaving the first stage stripping settler passes first to a surge tank where initial separation of entrained organic material takes place and then to the solvent recovery section where flotation units or coalescence beds are used to separate the bulk of the remaining entrained organic solvent. It then enters the electrowinning plant via a heat exchanger where it is heated by the spent electrolyte which itself is returning to the second stage stripping mixer-settler.
- Review on Some Past solvent extraction Work
- Factors Affecting the Cost and Performance of a Mixer-Settler
- Mixer Configuration
- Impeller Speed
- Residence Time
- Phase Continuity
- Organic/Aqueous Ratio
- Specific Settler Flow
- Settler Configuration
- Solvent extraction process conclusion
Advantages of Copper Solvent Extraction
Compared with cementation, solvent extraction has considerable advantages.
First of all, typical operating costs for a combined solvent extraction electrowinning operation can be as low as $30/ton of copper for the larger capacity plants. For cement copper, freight and smelting charges alone usually exceed this figure.
In solvent extraction the acid used in the leaching of the copper values is regenerated, allowing the raffinate to return to the leaching operation with a high useful acid content. This compares very favourably with cementation where the copper values are replaced by iron and no regeneration of acid occurs to enhance the free acid content of the spent liquor.
Most important of all is the fact that solvent extraction enables an operator to produce high grade cathode grade copper on site whereas cement copper always needs further treatment in a smelter. With pollution authorities frowning on effluents from smelters, it will become more and more difficult to find the required smelter capacity for this duty.
Contacting Equipment
We have shown, mixer-settlers as the contacting equipment on the flowsheet and it will be apparent that these devices have some quite important disadvantages.
For instance, for a mixer-settler treating 6000 IGPM of leach liquor at an organic/aqueous flow ratio of 1/1, the settler required is 120 ft long by 50 ft wide for abnormal LIX system. A normal plant will require at least 3 extraction stages and 2 strip stages so that the plot area involved for the complete installation is very large indeed. It also means that the initial solvent inventory is very high and the mixer-settlers plus inventory can contribute as much as 40% to the f.o.b. value of the plant and account for a high proportion of the erection and civil costs.
There are numerous other types of contactor, notably of the column type used in solvent extraction operations but unfortunately they do not lend themselves easily to copper solvent extraction where a high mixing contact time of the order of 2 to 3 minutes is required. Also the column contactors are very sensitive indeed to upsets in the system, their performance being very unpredictable at varying throughputs. In the mixer-settler the duties of mixing, where most of the mass transfer occurs, and phase separation are separated and thus the device lends itself more easily to scale up and to isolation of the practical problems which can arise.
The presence of solid materials which can build up in metallurgical systems is particularly disadvantageous in column contactors. The source of these solids may be:
- bacteria which can be either airborne or leach liquor borne, and whose breeding is promoted by the favourable conditions prevailing at the organic/aqueous interface,
- the spores of fungae such as Cladosporium Resinae which are frequently present in the kerosene carrier, and which also find conditions suitable for growth at the phase interface and
- very fine solid particles, entering the plant in the leach liquor. The fungal growth acts as a breeding ground for the bacteria and as a net to trap solid material. A solid layer can thus be formed at the interface, which can, because of its effect on settling rates, lead to “flooding” of the contactor.
In analysing systems in the laboratory the possibility of solids build-up must be examined and remedies for the particular situation found. Mixer-settlers are more suitable than column contactors for the study of the effects of the buildup of solids because they lend themselves to the separate study of mixing, mass transfer, settling and separation of the phases and the effect of solids on each operation can be examined. Also the design of a mixer-settler makes it easy to remove solids from the interface should any build-up occur.
It is possible, of course, to take preventative measures such as filtering the aqueous feed, and using microbiological filters on the organic feed, but even so it is not feasible to ensure perfect cleaning of the system in an industrial environment and the presence of traces of bacteria or fungae will tend to provide sources of infection which could lead to “crud” formation. Thus, for the present time at least, the most practicable contacting apparatus for large scale copper extraction plant is the mixer-settler.
- Parameters Provided by DPG Research & Development Division
- Isotherm Prediction from CUAD Curve
- Prediction of Extraction Isotherm
- Prediction of Strip Isotherm
- Interpretation of Equilibrium Isotherms
- Interpretation of the Equilibrium Isotherms
- Use of Equilibrium Isotherms taking into account single stage efficiencies.
- Use of Equilibrium Isotherms using Overall Efficiency
- Development of SX Design Parameters
Nickel, Cobalt, and Zinc Recovery by Solvent Extraction
The application of solvent extraction to copper recovery can now be considered an established, commercially viable process. This position has not yet been reached for the recovery of the other major non-ferrous metals in which we are interested.
The breakthrough for the copper process came with the development of a specific solvent, LIX, and up to now no equivalent product has been developed for nickel, cobalt, or zinc. However, the solvent manufacturing companies are putting a great deal of effort into research in these fields and we can expect to see new extractants in the not too distant future.
The extractants which are available for these metals are of the strongly pH dependent type, where the extractant is selective for the particular metal over a very narrow band of pH. Fig.2 gives an idea of what this means. For instance if we use Versatic 911 to extract copper from a solution of pH, the simplest duty for LIX-64N, we find we have to adjust the pH of the solution to about 3.5 to extract the major part of the copper. However, we would also pick up all the ferric iron and if we adjusted the pH to around 4 to pick up all the copper, we would pick up ferrous iron.
Looking at the zinc, nickel and cobalt extractions you will see that a simple extraction from a solution containing all three metal ions would not enable a separation to be effected since the extraction curves are too close together. So it is not a straightforward matter to apply solvent extraction in this case.
Currently we are working on a system which can separate nickel and cobalt by a multi-contact operation and initial results show some promise.
Depending on the components of the feed solution, Versatic 911 can find useful simple applications, of course. If we had a solution containing nickel and copper and traces of other impurities such as aluminium the copper could be extracted using LIX leaving the nickel to be removed by Versatic.
The cost of Versatic is only about $250/ton compared with $2000/ton for LIX so the use of Versatic is preferable wherever possible.
The particular property of Versatic 911 which interest us is its resistance to break-down in reducing conditions. If we extract copper, nickel, or cobalt into a solution of Versatic and then treat the loaded organic solution with a reducing gas at elevated temperature and pressure, the metal is precipitated as powder and the organic solution is regenerated. We have applied this technique to the production of copper and nickel powders with some success. For one client, we examined a means of recovering pure nickel from a solution containing nickel and zinc, normally a difficult separation. The process we adopted was to take both the nickel and the zinc into Versatic and applied reducing conditions. The nickel precipitated from the solution as nickel powder and the zinc remained unreduced. Washing the spent reduction liquor with sulphuric acid liberated the zinc as a zinc sulphate solution and rendered the Versatic suitable for re-use as an extractant.
Conclusion
Solvent extraction applied to copper recovery is a commercially viable operation due to the development of specific solvents. Power-Gas has backed up the theoretical data on the solvents with considerable development work in the examination of new applications for the solvents and the effect of differing operating conditions on plant design, in particular with respect to mixer-settler design and solvent recovery.
As a result, we are in a strong position to engineer plants for any application of the specific copper extractants.
For the other non-ferrous metals, development work is at an earlier stage but it is hoped that specific instances will arise where existing know-how can be applied in major plant design.
For more specific details about solvent extraction applied to copper recovery I refer you to a paper recently published in World Mining and now available as a brochure within P.G.C. “The Economics of Solvent Extraction Techniques applied to Copper Recovery”.


The Economics of
Solvent Extraction Techniques Applied to Copper Recovery
Solvent Extraction Glossary
Design of Large Scale Mixer-Settlers
https://www.911metallurgist.com/solvent-extraction-sx/
