Table of Contents
In the commercial dump leaching of copper, two methods have been generally accepted as a means for recovering copper from pregnant leach liquors.
The oldest and most widely used technique is copper cementation with scrap iron. The newer technique involves solvent extraction and electrowinning. As part of a program to assure adequate domestic supplies of essential metals, the Bureau of Mines, U.S. Department of the Interior, conducted comparative tests of leaching-cementation and leaching-solvent extraction-electrowinning. The objective of this research is to supply information for improving copper recoveries from copper leach dumps, thus extending and conserving copper reserves.
One of the major problems encountered in dump leaching is the precipitation of iron salts that render the ore impermeable to leaching solutions. This condition decreases the copper extraction rate and may eventually produce a dormant dump that may leave much undissolved copper within the ore bed. In addition to iron dissolved from the ore during leaching, iron derived from the cementation step may significantly contribute to the plugging of a leach dump. The newer solvent extraction-electrowinning method recovers copper from leach solutions without adding iron to the leach system.
Although economic comparisons between cementation and solvent extraction-electrowinning techniques have been reported, the results of studies that compare effects of the two recovery techniques on leaching have not been reported. The Bureau of Mines, aware of the problems associated with maintaining leach dump permeability, has reported the beneficial effects of removing ore fines prior to leaching and the enhancement of the copper leaching rate by injecting gaseous oxygen into a nearly dormant leaching system. To supply further information concerning copper dump leaching, this report describes the results of leach-cementation and leach-solvent extraction-electrowinning procedures, using 6- to 7-tonne ore samples.
Materials and Methods
Ore Sample Description
Low-grade Montana open pit mine waste containing about 0.25 percent copper was used in the leach testing. The host rock was an altered quartz monzonite containing about 5 weight-percent pyrite. The copper content was distributed among several minerals with chalcocite predominating. The distribution in weight-percent was 66 chalcocite-digenite, 17 chalcopyrite, 11 covellite, and 6 enargite.
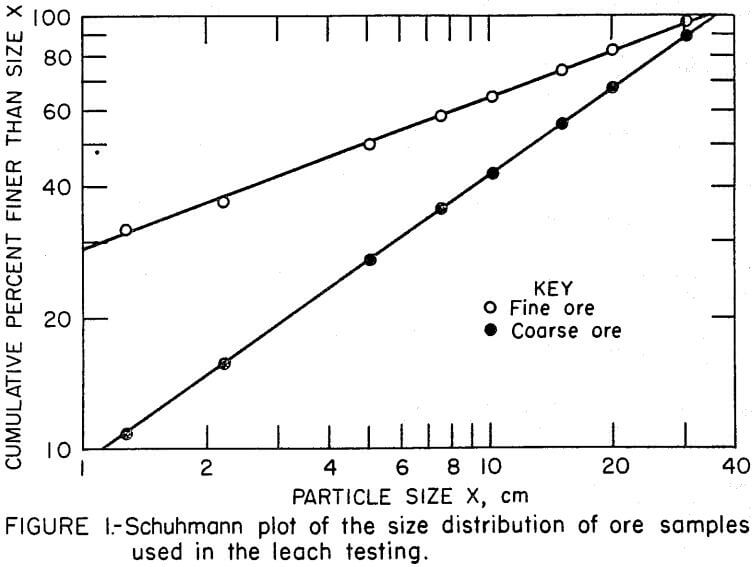
One objective of the investigation was to determine the effect of the amount of minus 1.27-cm fines on bed permeability using the alternate copper recovery techniques. Two comparative tests were made with samples that contained 11 percent minus 1.27-cm fines, and another comparison was made with samples that contained 32 percent minus 1.27-cm fines. The samples were prepared by combining various size fractions of the ore sample to yield the particle size distributions shown in Figure 1. The top size of each sample was 37 cm. Each sample containing 11 percent minus 1.27-cm fines whereas the samples containing a higher proportion of fines assayed 0. 26 percent copper.
Leaching Procedure
The ore samples were leached in glass-fiber-reinforced polyester columns 1.37 m in diameter by 3.05 m high. For the comparison with ore containing 11 percent fines (minus 1.27 cm) 6800-kg samples were used; for the comparison with ore containing 32 percent fines, 6410-kg samples were used. Leach solution with a pH of 2.0 was pumped to the top of the ore at an initial rate of 3000 l/m² d and uniformly distributed over the ore. The solution percolated downward through the ore, was collected in a surge tank, and recirculated back to the top of the ore until the copper content was 1 to 2 g/l. The pumping was then stopped and the soluble copper recovered by the appropriate method. The Barren solution was acidulated to a pH of 2.0 with sulfuric acid and used to leach additional copper.
Copper Recovery Procedures
Copper recovery by cementation was accomplished in a glass column 15 cm in diameter by 2.3 m high containing shredded detinned iron scrap. Pregnant solution was pumped upward through the iron. Sulfuric acid was added to the solution prior to passage through column to maintain an effluent pH of 2.0 to prevent precipitation of iron salts.
The solvent extraction recovery system was comprised of a four-stage countercurrent mixer-settler unit for extraction followed by a four- stage countercurrent stripping unit and an electrowinning cell. The organic extractant consisted of 10 volume-percent LIX-64N in kerosine. The solvent extraction system was operated with an organic flow rate of 0.7 l/min and an aqueous flow of 0.9 l/min. The aqueous feed to the solvent extraction section contained 1 to 2 g/l of Cu and raffinate containing less than 0.1 g/l of Cu was returned to the leaching circuit. The solvent was loaded to nearly 1.5 g/l of Cu; stripping reduced the copper concentration to 0.1 g/l of Cu. The stripping and electrowinning units were operated to maintain a balanced copper extraction unit. The electrolyte used in the stripping unit was circulated at 0.15 l/min and contained 20 to 30 g/l of Cu and 150 g/l of H2SO4. The electrowinning cell was operated at a current density of 172 A/m².
Experimental Results
Leaching the Coarse Ore
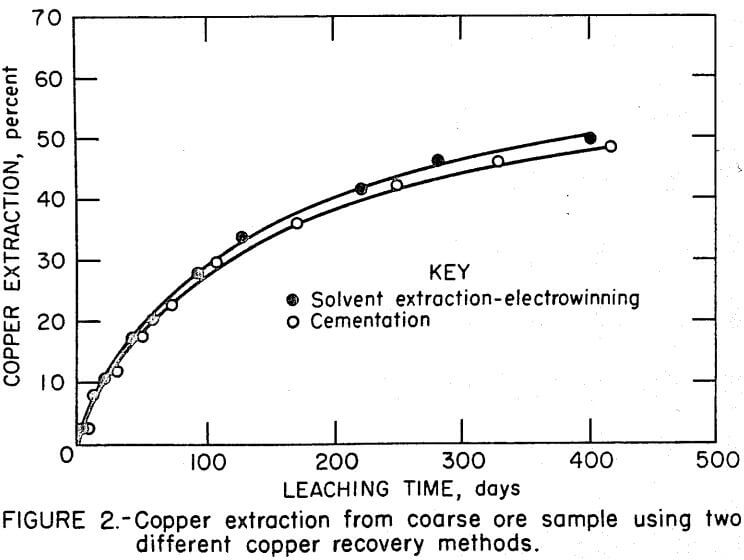
The two coarse ore samples (11 percent minus 1.27 cm) were leached for 416 days. Copper was recovered from solution 32 times in each leach test. Copper extractions, based on leach solution and ore residue assays, are shown in Figure 2. After 416 days, copper extraction was 48 per-cent for the leach-cementation test and 51 percent for the leach-solvent extraction-electrowinning test.
Acid addition to maintain the leach solution at pH 2.0 for the leach-cementation test was 7.5 kg H2SO4/t of ore; whereas, the leach-solvent extraction-electrowinning test required only 4.6 kg H2SO4/t of ore.
The iron concentration increased in the leach solutions of both tests, but the rate of increase was more rapid in the test using cementation. After 131 days of leaching, the iron concentrations were 31 g/l in the leach-cementation test solution and 14 g/l in the leach-solvent extraction-electrowinning test solution. Beyond 131 days, the iron concentrations were maintained at 10 g/l by bleeding solution from each system and replacing it with water. This procedure simulates large-scale leaching practice in which dissolved iron is controlled by oxidation and settling in ponds.
In the leach-cementation test, the amount of iron added to the system during the cementation operations was 6.15 kg/t of ore and 3 kg of iron per tonne of ore was bled from the system. At the completion of the test, only 0.35 kg of iron per tonne of ore remained in solution. Based on these amounts, 2.8 kg of iron per tonne of ore accumulated in the ore. However, the accumulated iron in form of precipitated salts was not in sufficient quantity to cause ponding of the ore in either test, and a solution flow rate of 3000 l/m² d was maintained throughout the test.
The presence of both iron- and sulfur-oxidizing bacteria in both leach solutions was verified by semiquantitative bacteriological tests. Good bacterial activity was evidenced in both tests by the low ferrous iron levels (less than 0.1 g/l) throughout the test period.
Leaching the Fine Ore
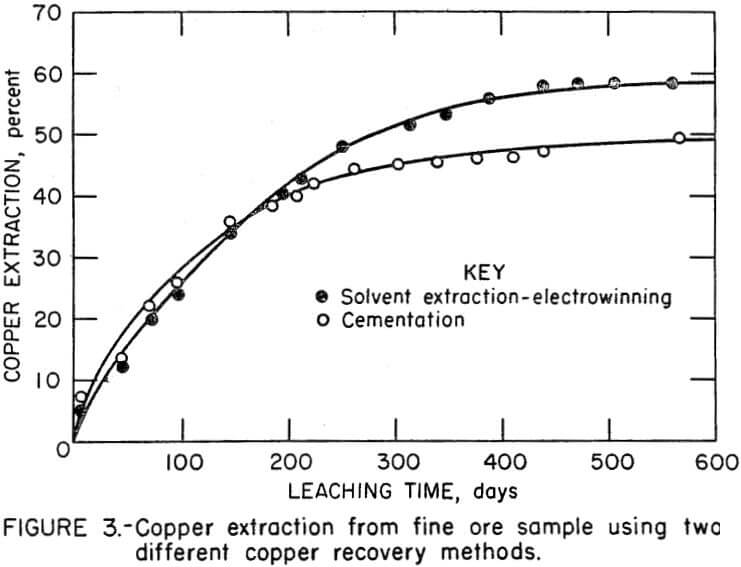
The two fine ore samples (32 percent minus 1.27 cm) were leached for 568 days during which time copper was recovered from solution 25 times. Copper extractions for the two tests are shown in Figure 3. At the completion of the tests, copper extraction was 60 percent for the leach-solvent extraction-electrowinning test and 48 percent for the leach-cementation test. Although similar extractions were achieved during the first 200 days, a noticeable difference in copper extraction was apparent after 300 days.
Acid addition to maintain the leach solution at pH 2 was 7.0 kg H2SO4/t of ore for the leach- cementation process and 2.9 kg H2SO4/t of ore for the leach-solvent extraction-electrowinning process.
Maximum total iron concentrations in leach liquors reached during 568 days of leaching were 20 g/l using cementation and only 10 g/l using solvent extraction. Leach liquor ferrous iron concentration remained at 0.1 g/l or less when solvent extraction was used. Using cementation, however, the ferrous iron content held at 0.1 g/l during most of the first 151 days of leaching and then increased to a level of 70 percent to 100 percent of the total iron content during the remainder of the test.
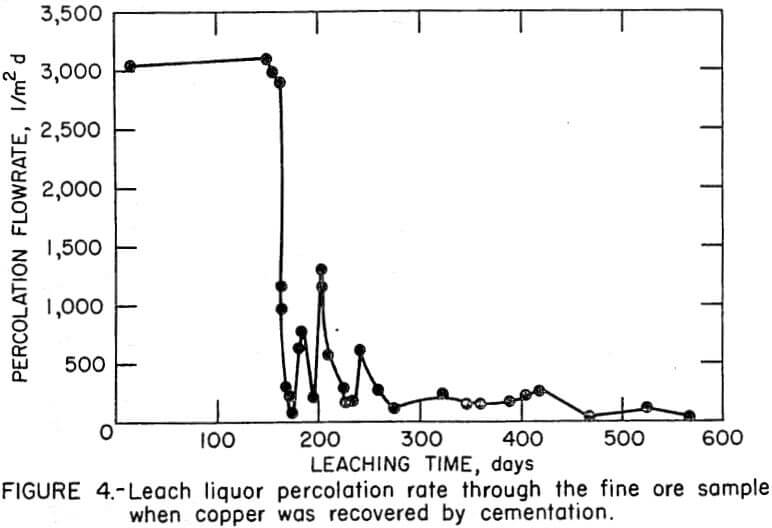
A leach solution percolation rate of 3000 l/m² d was maintained when solvent extraction was used; however, the percolation rate decreased to only 77 l/m² d after 162 days when cementation was used. Precipitation of iron compounds from the high-iron leach liquor in the leach-cementation test impeded the percolation rate through the fine-ore bed and the cyclic nature of the percolation rate is shown in Figure 4.
Copper extraction became retarded when the ore contained 32 percent minus 1.27-cm fines and copper was removed by cementation. Decreased copper extraction was attributed to two related factors. Leach solution percolation rate was greatly decreased by blinding of the bed with precipitated iron compounds, and the concentration of ferric iron, a solvent for the copper sulfide minerals, decreased. The oxidation of ferrous to ferric iron largely was due to the action of aerobic bacteria within the bed and poor bed permeability caused oxygen starvation that dimished bacterial action. This conclusion was reached when gas samples from a perforated tube embedded in the ore were taken on the 150th day of leaching. Gas analyses showed oxygen contents of 20 and 4.2 percent for the leach-solvent extraction and leach-cementation tests, respectively. Thus, copper removal by solvent extraction enabled better copper leaching when the ore contained a large proportion of fines.
Consumption of sulfuric acid was least when solvent extraction was used because acid consumption was related to the relatively large amount of iron dissolved during cementation. Sulfuric acid was used by the reaction of iron and acid, equation 1, and by the oxidation of ferrous iron, equation 2.
H2SO4 + Fe → FeSO4 + H2……………………………..(1)
2FeSO4 + H2SO4 + ½O2 → Fe2(SO4)3 + H2O…………………………..(2)
Less acid was used when leaching the fine ore, and this was believed to result from leaching the fines that contained a higher proportion of friable iron sulfide minerals than the harder, coarse material. The dissolution of pyrite, for example, is shown by equation 3; this reaction plus the oxidation reaction 2 gives the net reaction 4.
FeS2 + 7Fe2 (SO4)3 + 8H2O → 15FeSO4 + 8H2SO4…………………..(3)
2FeS2 + 7-½ O2 + H2O → Fe2 (SO4)3 + H2SO4………………………….(4)
Comparative leaching tests were made to determine copper extractions from low-grade chalcocitic ore using either cementation or solvent extraction-electrowinning methods to recover copper from the acidic leach liquors. Two minus 37-cm ore samples that had different particle size distributions were tested; one sample contained 11 percent minus 1.27-cm fines, and the other had 32 percent minus 1.27-cm fines.
The copper recovery system had little, if any, effect on copper extraction using the relatively coarse ore sample that contained 11 percent minus 1.27- cm fines. After 416 days of leaching, copper extractions were 48 percent for the leach-cementation test and 51 percent for the leach-solvent extraction-electrowinning test.
In comparative tests using the ore sample that contained 32 percent minus 1.27-cm material, the solvent extraction-electrowinning copper recovery system yielded the highest copper extraction. After 568 days of leaching, copper extractions were 48 percent for the leach-cementation test and 60 percent for the leach-solvent extraction-electrowinning test. Cementation of the copper, unlike solvent extraction-electrowinning, contributed additional iron to the leach column, which resulted in the precipitation of iron compounds that decreased ore permeability. The eventual plugging of the ore bed resulted in low oxygen content within the column, poor bacterial activity, and low ferric iron concentrations. These factors led to a slow copper leaching rate.
Consumption of sulfuric acid, necessary to maintain a pH of 2, was minimized by using solvent extraction-electrowinning for copper recovery. The leach-cementation test required more acid because acid was consumed by the dissolution of metallic iron during cementation and by the oxidation of the resulting ferrous iron.
 |
 |
 |
 |
To assist in fulfilling the Bureau of Mines goals of maintaining adequate mineral supplies to meet national economic and strategic needs, and to minimize the environmental impacts associated with mineral-processing operations, a leaching-solvent extraction-electrowinning technique was compared with a leaching-cementation technique for processing low-grade copper ores. Large-scale laboratory column leaching tests (6 to 7 tonnes) were conducted on minus 37-cm chalcocitic ore using ferric sulfate leach solutions at 25° C. Two columns contained 11 percent minus 1.27- cm fines, and two columns contained 32 percent minus 1.27-cm fines. After 416 days of leaching ore samples containing 11 percent minus 1.27- cm fines, copper extraction was 48 and 51 percent for the leach-cementation and leach- solvent extraction-electrowinning procedures, respectively. When leaching ore samples containing 32 percent minus 1.27-cm fines, the leaching- solvent extraction-electrowinning technique extracted 60 percent of the copper after 568 days, whereas the leaching-cementation technique extracted only 48 percent of the copper. In the latter test, leach solution ponded on the ore, which reflected a decrease in ore column permeability. The decreased permeability was attributed to the generation of excessive amounts of iron salts during cementation; the salts precipitated on the fine ore particles and in the interstices, thus plugging the column. This decrease also lowered the available oxygen, resulting in poor aerobic bacterial activity which slowed the oxidation rate of ferrous ions to ferric ions. Sulfuric acid required to maintain a leachant pH of 2 was nearly halved when copper was recovered by solvent extraction and electrowinning rather than by cementation.
