It has been reported that chalcopyrite can be floated selectively from an ore containing pyrite, sphalerite, and silicious gangue minerals without using a collector. Sodium sulfide was used as a promoter, presumably to clean the chalcopyrite surface of its hydrophilic oxidation products. Later it was found that the potential of the pulp, measured with a platinum-calomel electrode pair, was critical for the success of collectorless flotation.
Experimental
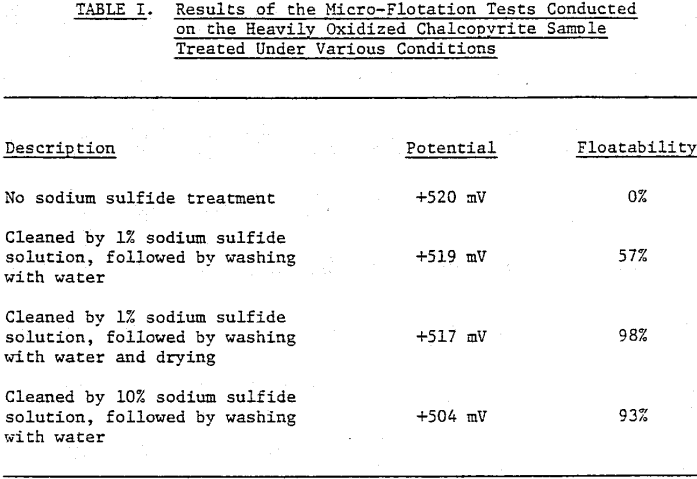
Most of the flotation tests were conducted using the freshly prepared chalcopyrite sample. However some tests were made using a chalcopyrite sample from the same deposit that was prepared four years prior to this study and stored in a dry atmosphere without precautions to prevent oxidation.
Reagent grade sodium sulfide (Na2S · 9H2O) and purified hydrazine hydrate (N2H4 · H2O) were used as reducing agents to control the potential in the micro-flotation experiments. No oxidizing agents were used to control the potential since the potential of the double-distilled water was high enough to give good flotation. Sodium sulfide was also used to clean the surface of the chalcopyrite samples.
Results
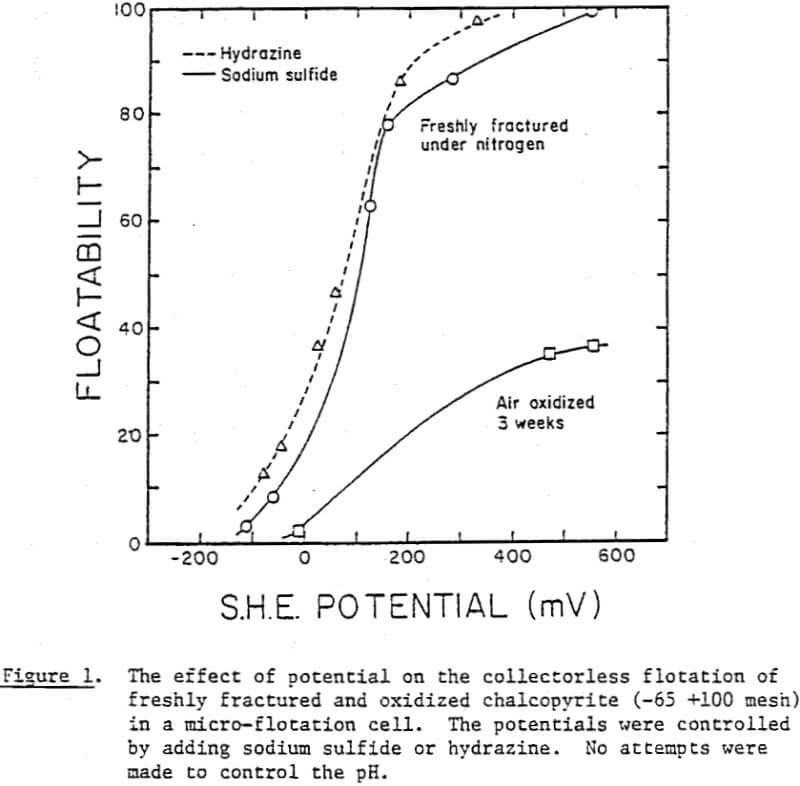
In one series, the potentials were controlled by adding varying amounts of sodium sulfide, and in the other, hydrazine was used. When no reducing agents were added, the potentials were above 300 mV, and almost complete flotation was possible. As the potentials were reduced, the floatability was reduced significantly in both series.
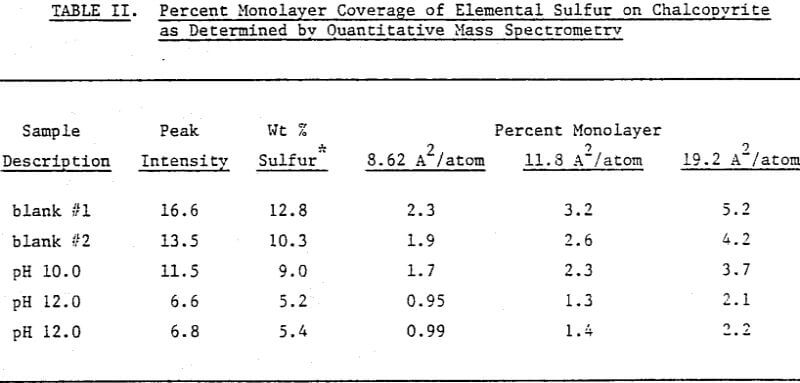
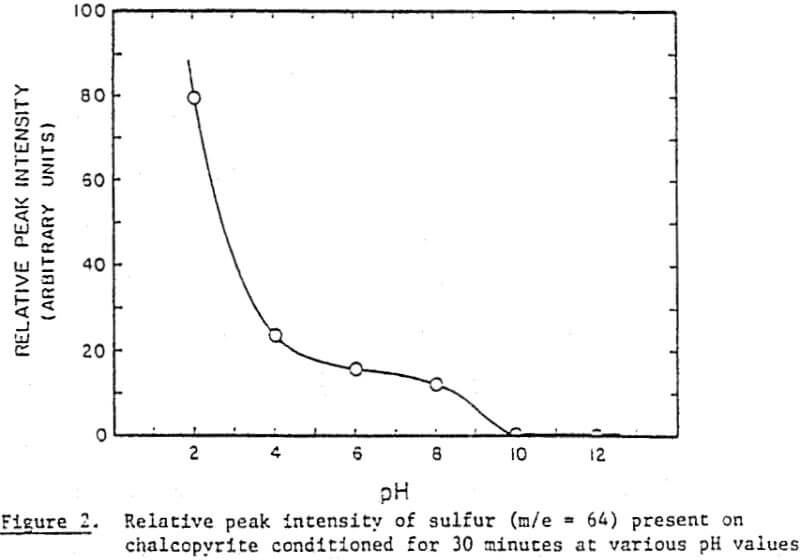
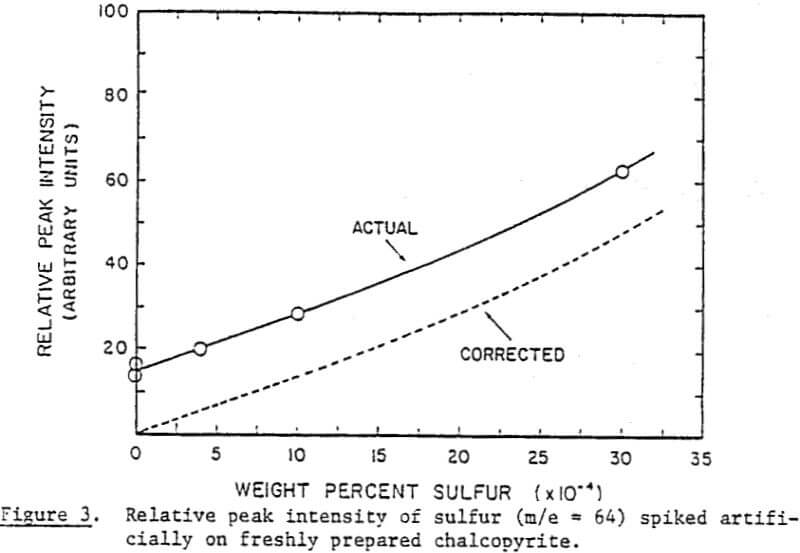
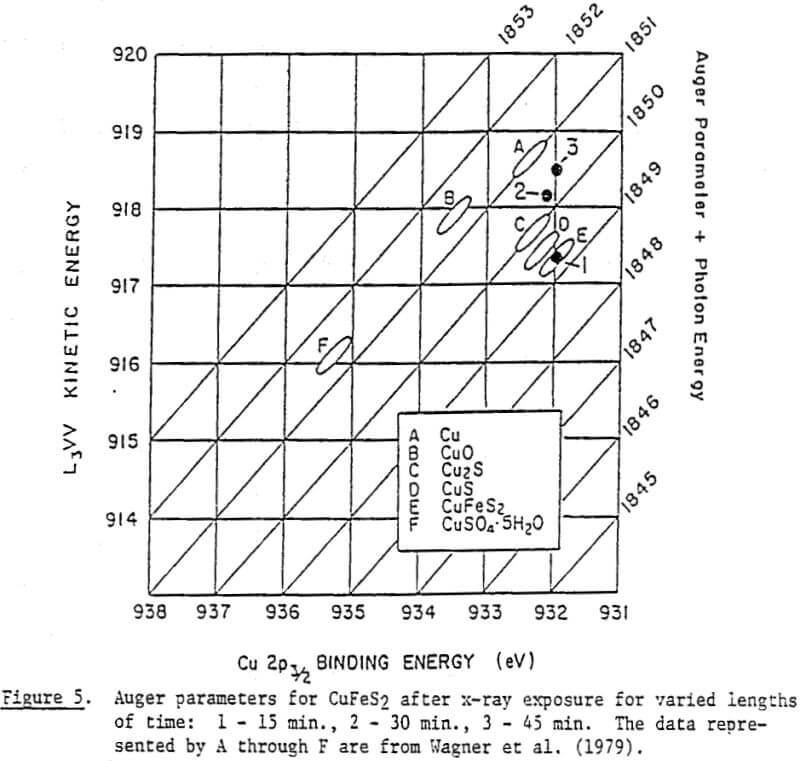
In order to determine the elemental sulfur in a quantitative manner, a calibration curve has been established. It has been constructed by spiking known amounts of elemental sulfur on chalcopyrite samples, as has already been described, and taking peak intensity readings at the maximum sensitivity setting. Note that the unspiked blank samples of chalcopyrite showed some sulfur.
Thus, a series of spectra was collected from a chalcopyrite sample over a period of approximately one hour. Both the copper 2p and copper Auger spectra were collected to identify the species using the Auger parameters. The results of this experiment. As indicated, Cu(I) was being reduced to copper metal after a prolonged exposure time. The sulfur and copper spectra collected during the same period were curve-resolved so that changes in the amounts of various species could be determined. However, the amount of elemental sulfur did not increase significantly while Cu(I) was being photoreduced.
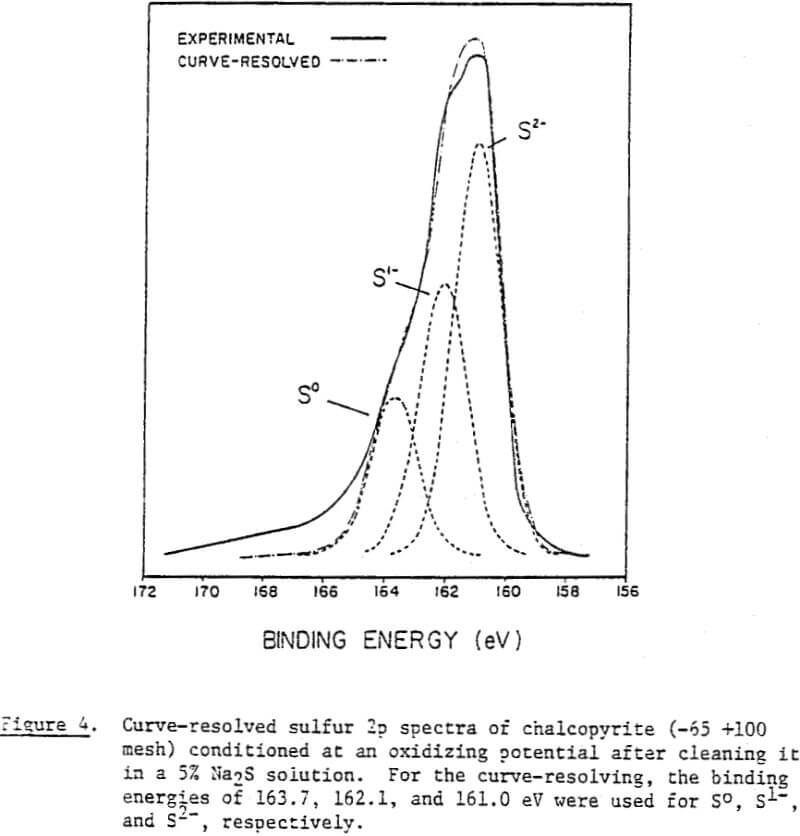
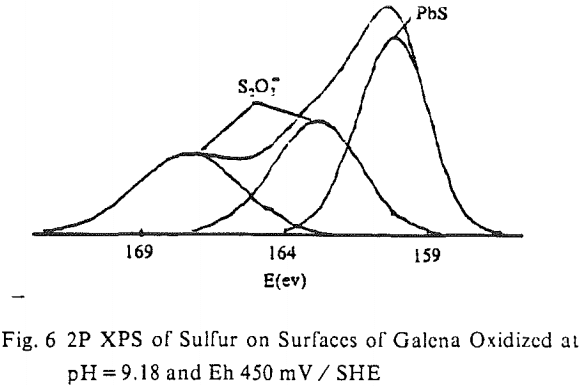
The surface species changing with potential have not been clearly identified, however. Thus, micro-flotation tests were conducted on pure samples of chalcopyrite conditioned at various potentials in the alkaline pH region, and the flotation products were analyzed by ESCA. Samples conditioned at -236 mV and +215 mV showed drastic differences in floatability (8% versus 77%). If the flotation is caused by elemental sulfur, one would expect to see a larger amount of elemental sulfur on the chalcopyrite sample conditioned at +215 mV. The reverse is found to be the case, however. The shoulder at approximately 164 eV, indicating the presence of elemental sulfur, is stronger at the potential of -236 mV than at +215 mV. A possible explanation might be that the sulfide ions added to reduce the potential may have been oxidized during the drying procedure to become elemental sulfur.
Discussion
The oxidation products formed on chalcopyrite at alkaline pH values are ferric hydroxide (Fe(OH)3), elemental sulfur (S°), and covellite (Cu¹+S¹-). It is difficult to confirm the presence of Fe(OH)3 by ESCA studies because Fe2p peaks are fairly diffuse and difficult to resolve. On the other hand, the presence of S¹- and S° has been clearly observed in the S2p spectra obtained. There is no evidence that the elemental sulfur detected is a result of photo-oxidation of S²- ions coupled with photoreduction of Cu (I).
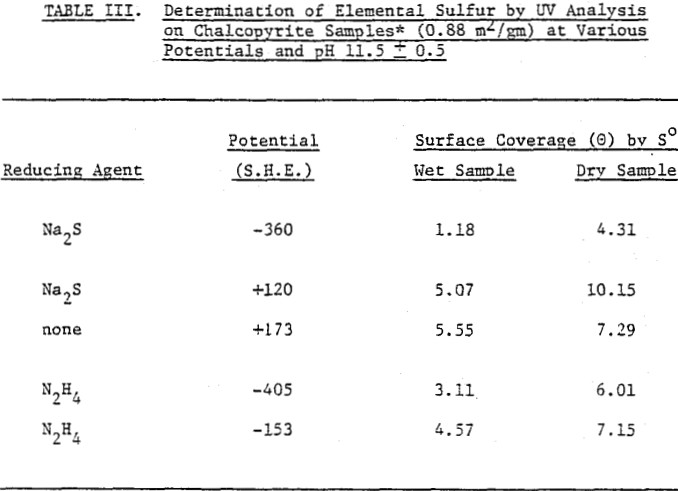
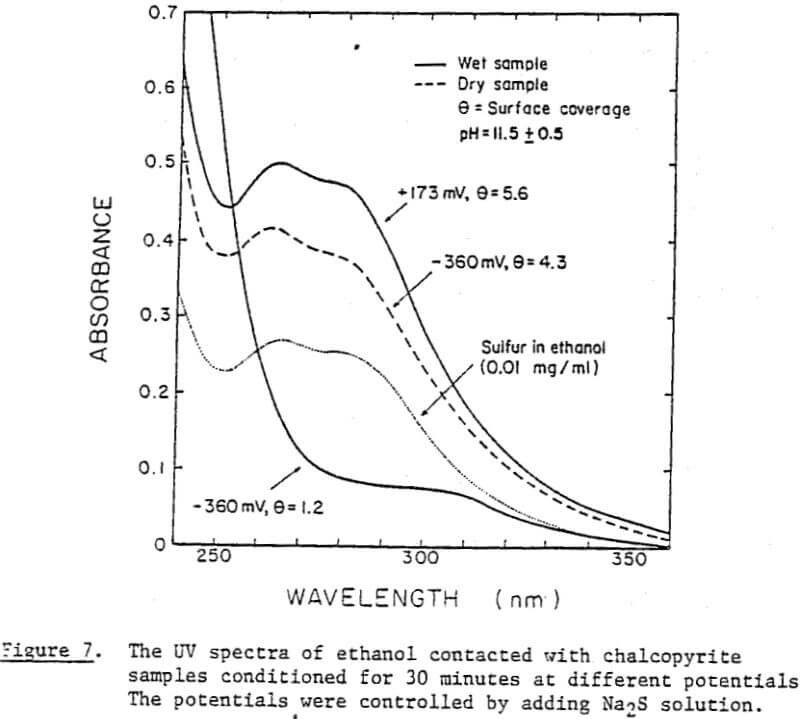
Perhaps the strongest evidence for elemental sulfur being present on the chalcopyrite surface and playing a decisive role in its collectorless flotation has been given by the UV analysis. The samples conditioned at oxidizing potentials showed a large amount of elemental sulfur, while those conditioned at reducing potentials showed a significantly smaller amount. Considering that collectorless flotation occurs only at oxidizing potentials, elemental sulfur may be the hydrophobic entity that is responsible for the flotation.
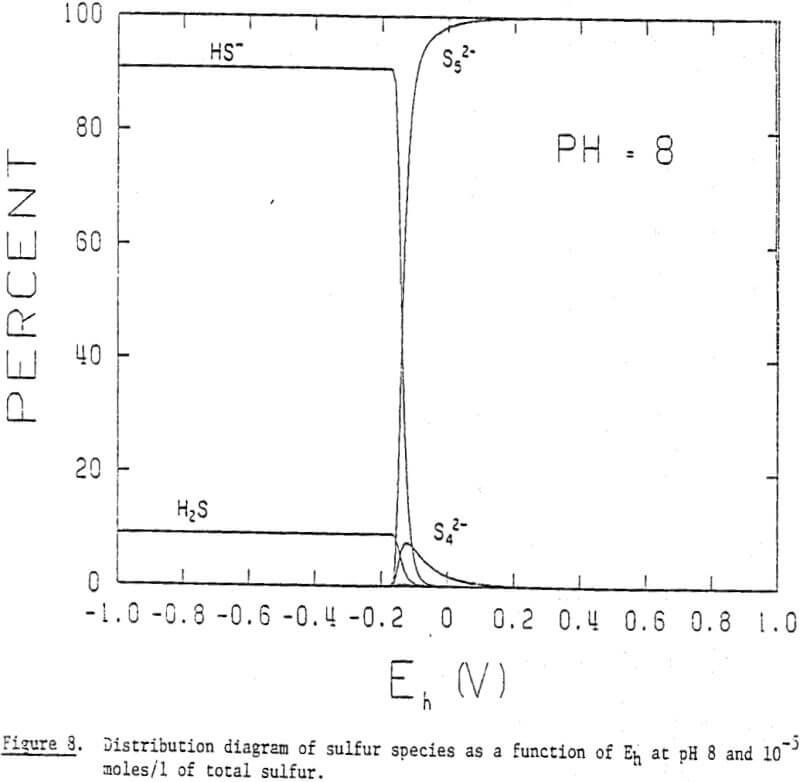
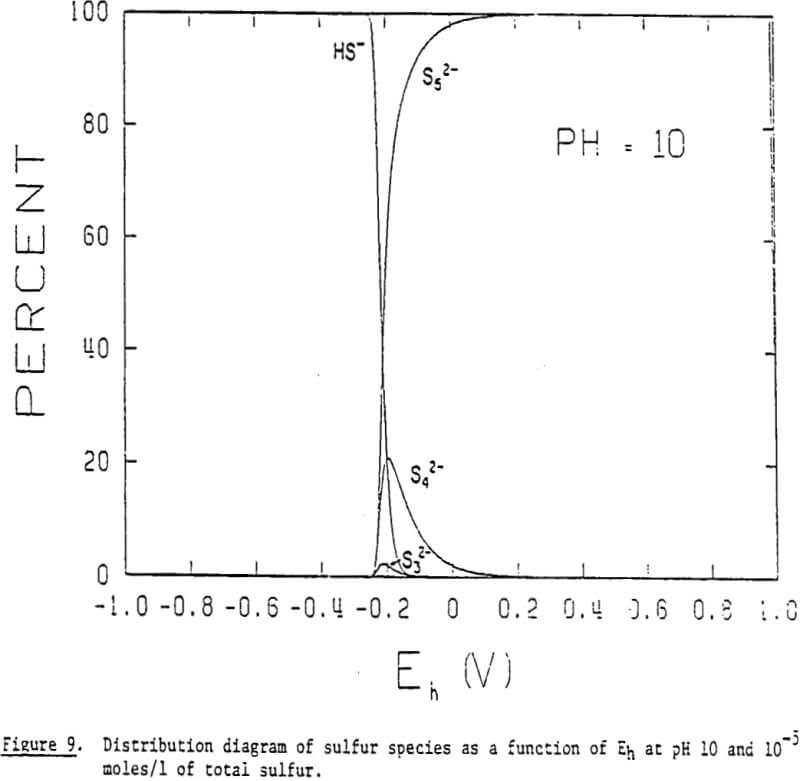
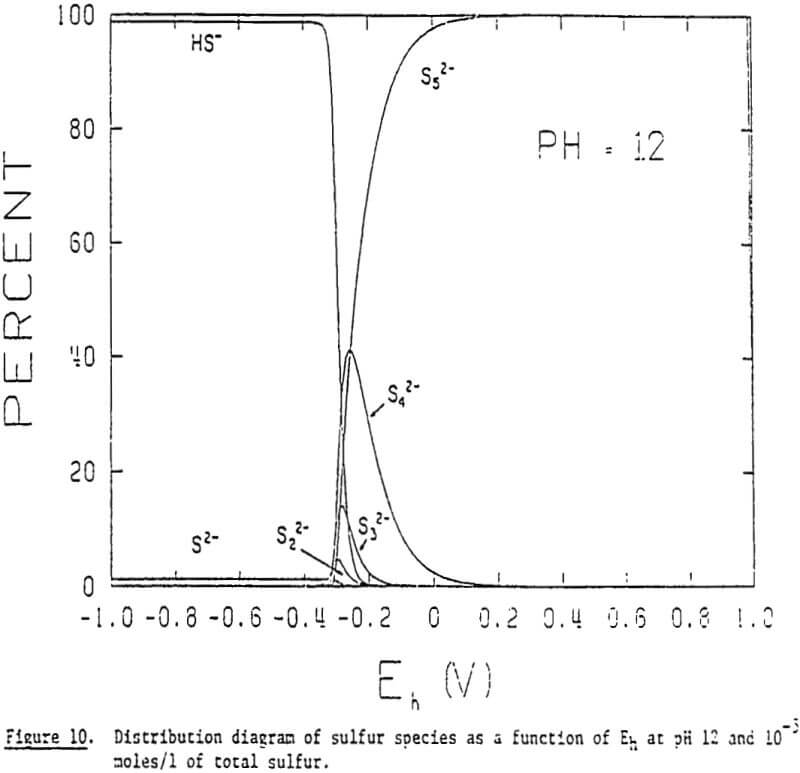
It is, therefore, possible that polysulfides may play an important role in the collectorless flotation of sulfide minerals in alkaline solutions. The general formula for polysulfides may be given as Sx, in which x represents the number of sulfur atoms in its chain structure. The two sulfur ions at both ends of a chain have a valence of -1 and those in the middle are in elemental form. Polysulfide ions adsorbed on a mineral surface would make it hydrophobic, much like elemental sulfur does in acidic solutions.
The distribution diagrams also show that longer chain polysulfides become predominant at higher potentials. One would expect that the longer the chain length of the adsorbing polysulfide, the more hydrophobic the mineral becomes. This may help explain the increase in floatability with increasing potential. In addition, the distribution diagrams show that the critical potential at which polysulfide formation begins to occur is lowered with increasing pH: -0.17 V at pH 8, -0.25 V at pH 10, and -0.32 V at pH 12. These findings indicate that the oxidation of sulfide to polysulfide is favored by raising the pH.
From the foregoing discussions, one might suppose that chalcopyrite is naturally hydrophilic and requires superficial oxidation for collectorless flotation. The oxidation product that renders the mineral hydrophobic is either elemental sulfur or polysulfide, depending on the pH and the extent of oxidation.

How Collectorless Flotation Works
The present studies have shown that most metallic sulfide minerals can be well separated in the collectorless flotation only within the range of a certain pulp potential (oxidizing potential), such as chalcopyrite, galena and sphalerite activated by Cu²+ ion, and have the collectorless floatability to a certain extent, such as pyrite, pyrrhotite, poikilite, perkerite, chalcocite and covellite etc., with the exception of molybdenite, eolite, arsenopyrite and stibnite with natural hydrophobicity. The sequence of floatable extent of sulfide minerals in the collectorless flotation is as follows: chalcopyrite, galena, pyrrhotite, covellite, poikilite, sphalerite, pyrite and arsenopyrite.
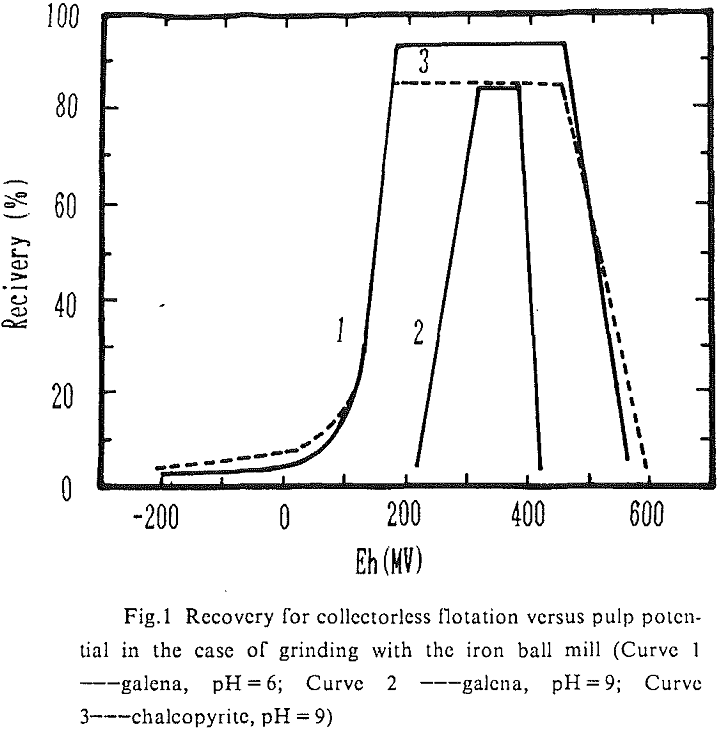
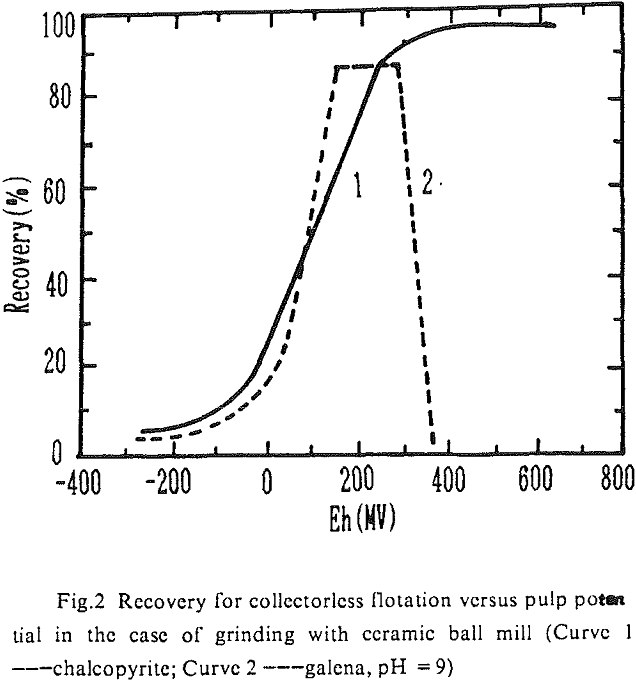
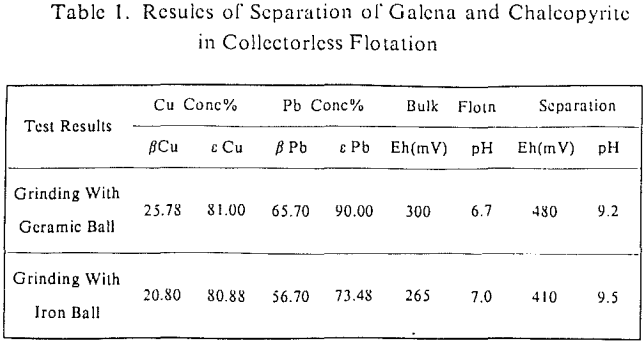
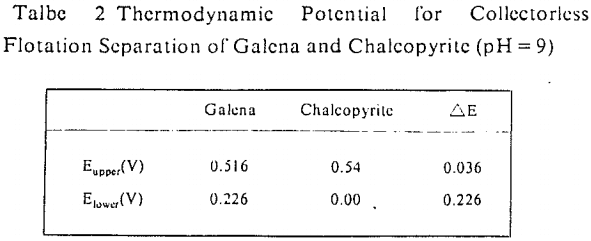
The influence factors lie in the grinding condition, pH value of flotation and the pulp potential. On conditions of grinding with ceramic ball mill and pH = 9, the lower limit of pulp potential was 50-100 mV and the upper limits 300 mV (galena) and 600 mV (chalcopyrite) in the collectorless flotation of these two kinds of minerals. In the case of grinding with iron ball mill and pH = 9, the pulp potential ranges which galena and chalcopyrite can be separated in the collectorless flotation were 280-400 mV and 150-500 mV individually. Obviously, there was a greater difference in the behavior of collectorless flotation of chalcopyrite and galena on conditions of alkaline medium and high oxidizing potential.
At pH = 9, the upper and lower limits of potential for the collectorless flotation of galena are determined by equation (1-2) individually:
PbS + 2H2O = Pb(OH)2 + S° + 2H+ + 2e……………………………………..(1)
PbS + 6H2O = Pb(OH)2 + SO4- + 10H+ + 8e………………………………(2)
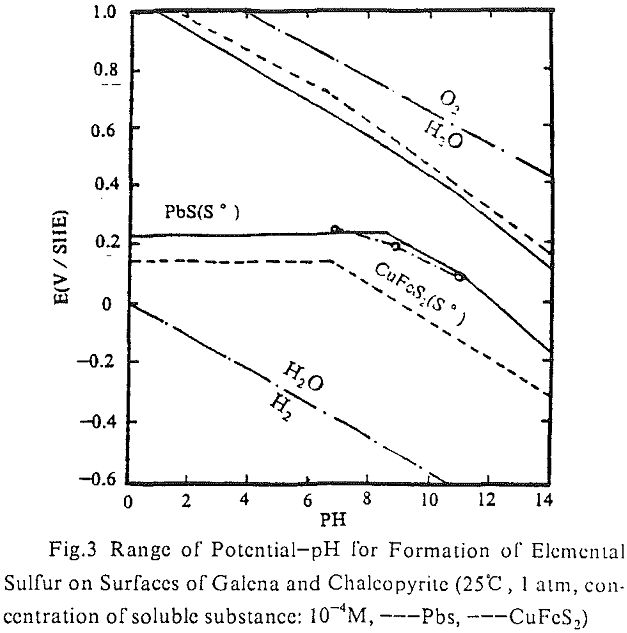
At pH = 9, the lower limit of potential for the collectorless flotation of chalcopyrite is determined by equation (3):
CuFeS2 + 3H2O = CuS + Fe(OH)3 + S° + 3H + + 3e…………………………………..(3)
CuS + 6H2O = Cu(OH)² + SO4- + 11H- + 8e……………………………………..(4)
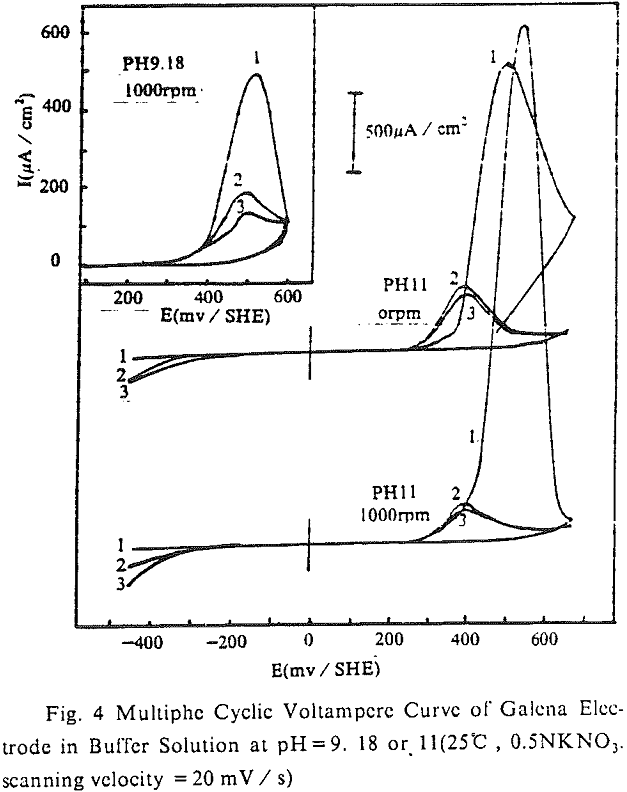
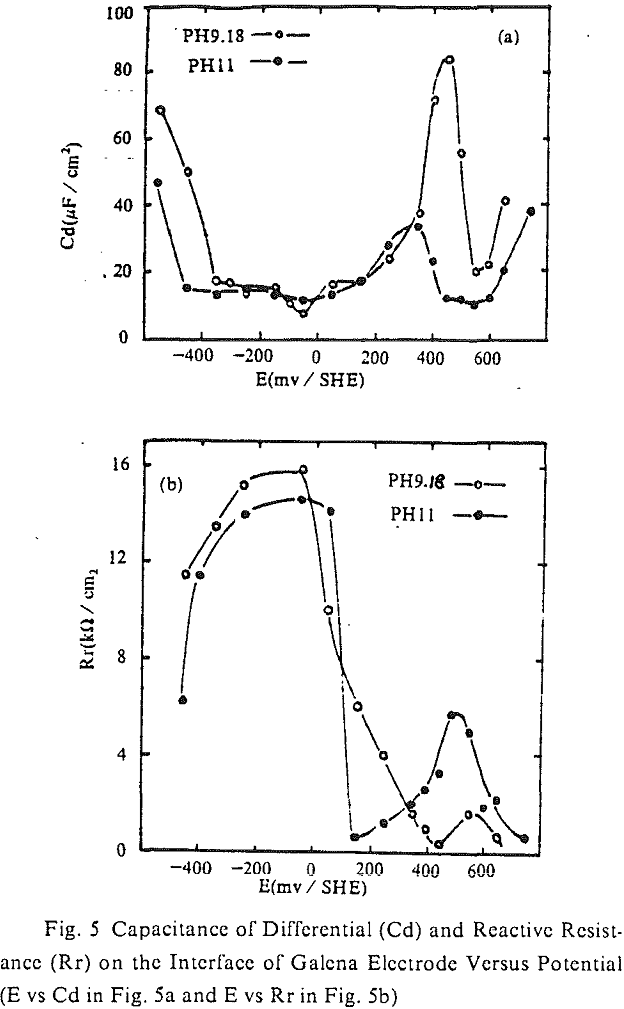
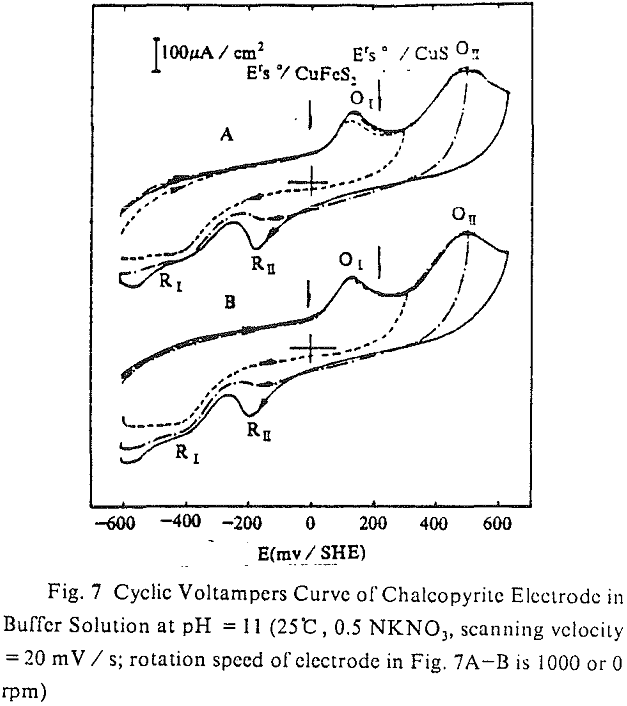
The cyclic voltampere curve (pH =11) of chalcopyrite electrode is shown. When the upper limit of scanning potential was 300 mV / SHE, there was a current peak of anode (O1) and cathode (R1) respectively, and each was corresponding to the reaction of positive or negative according to equation (3). After limit of scanning potential was increased by 500 mV / SHE, the second current peak of anode (O11) or cathode (R11) was produced individually. The second current peak of anode originated from the continuous oxidation of CuS, that was from the first oxidizing reaction of anode (equation (3)), at high potential.