The selective flotation of alkali halide salts such as KCl and NaCl is of great industrial significance and has been considered by flotation chemists for many decades.
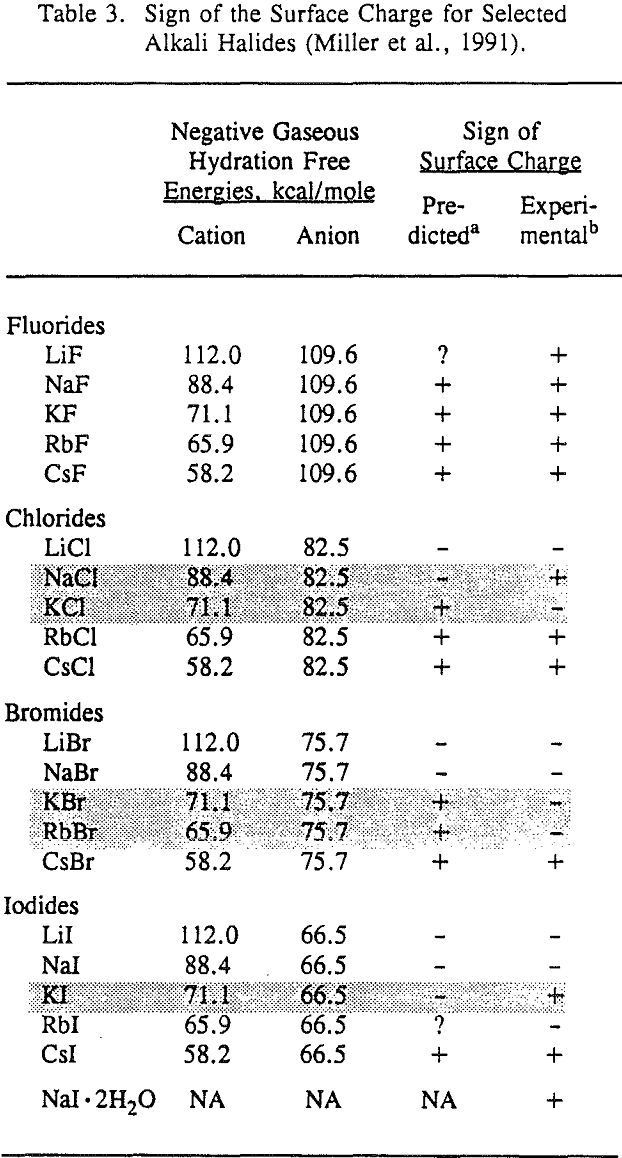
The surface-charge model was rather controversial when it was first presented at the SME Annual Meeting in Los Angeles in 1967. Basically this model suggested that the alkali halide salts were charged in their saturated brines and that collectors adsorbed as ions or neutral molecular dipoles depending on the specific flotation system under consideration. The model was quite speculative, with the sign of the surface charge being predicted from simplified lattice ion hydration theory. Little direct experimental evidence was given to support the predicted surface charge, and identification of surface collector species was not reported at all.
Experimental
Ultrapure alkali halides (>99.9%) used in this work were purchased from Alfa Research Chemicals. The collectors dodecylamine hydrochloride and sodium laurate were obtained from Kodak Research Chemicals and were used as received. Milli Q water (18 MΩ) was used in all experiments. Glassware used in the experiments was soaked in chromic acid, rinsed with pure water, soaked in 7 M KOH, rinsed in pure water, and dried just prior to use.
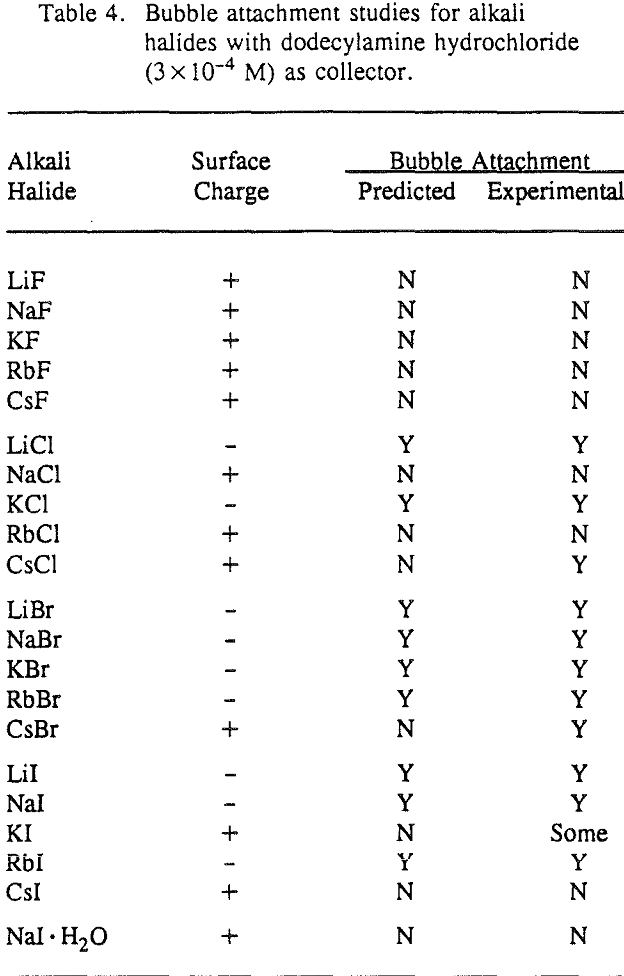
Bubble attachment experiments were completed using an electronic induction timer (Mineral and Coal Technologies, Virginia). Particles (100×150 mesh) of twenty-one alkali halides were conditioned in their respective saturated solutions in order to establish equilibrium. The collector colloid was prepared in the corresponding brine (3×10 -4 M dodecylamine hydrochloride) and then added to the alkali halide system and conditioned for an additional five minutes.
Experimental Results and Discussion
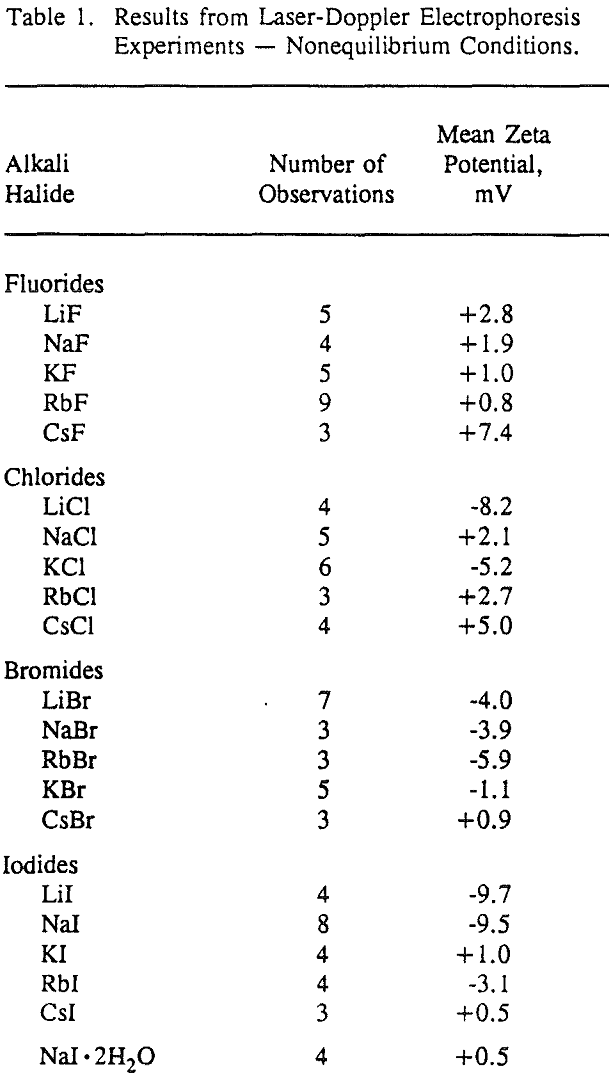
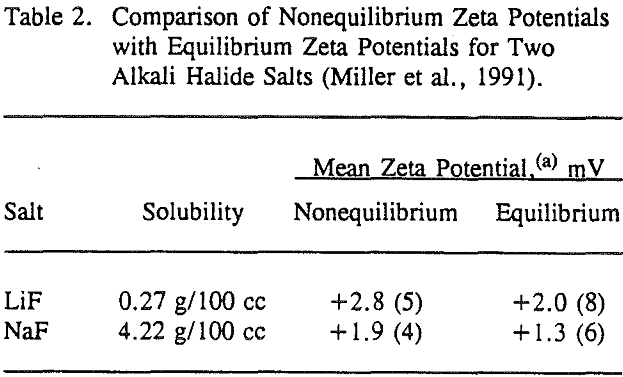
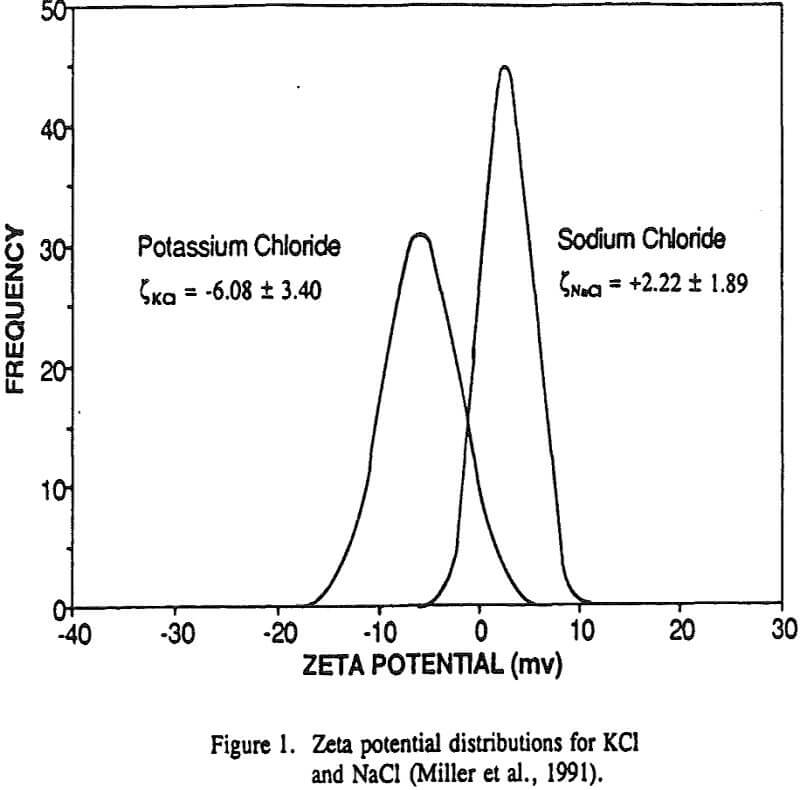
The validity of these nonequilibrium measurements was demonstrated by making equilibrium measurements for LiF and NaF. The nonequilibrium zeta potentials to be equivalent in sign to the equilibrium zeta potentials measured after one hour of equilibration between the salt and the aqueous solution. Most importantly, these nonequilibrium zeta potential measurements can be used to establish the sign of the surface charge for each alkali halide salt, and these results are generally confirmed by lattice ion hydration theory.
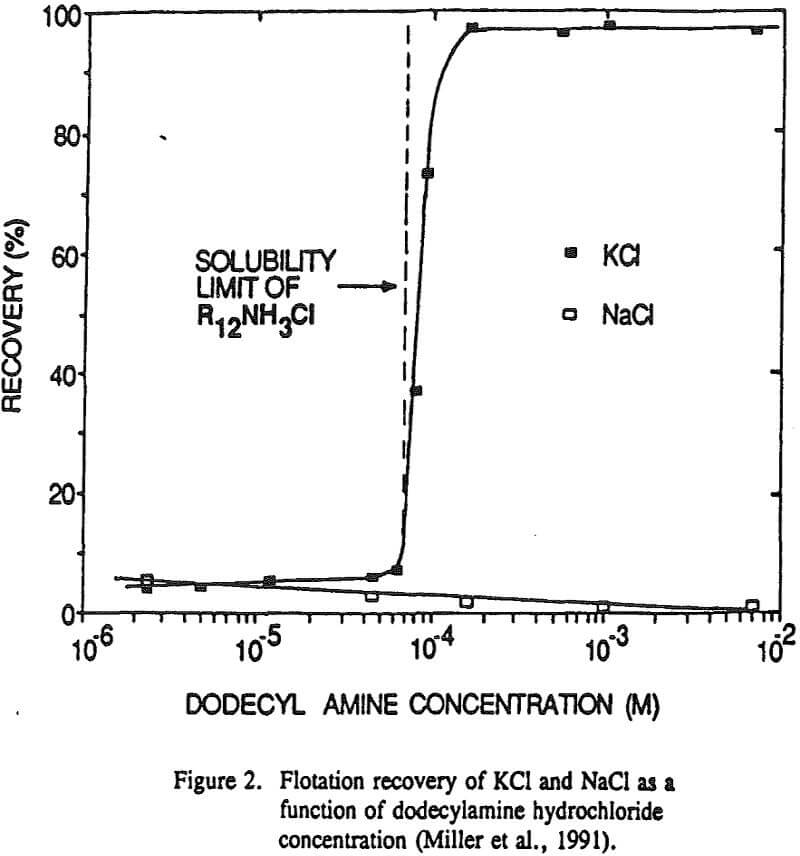
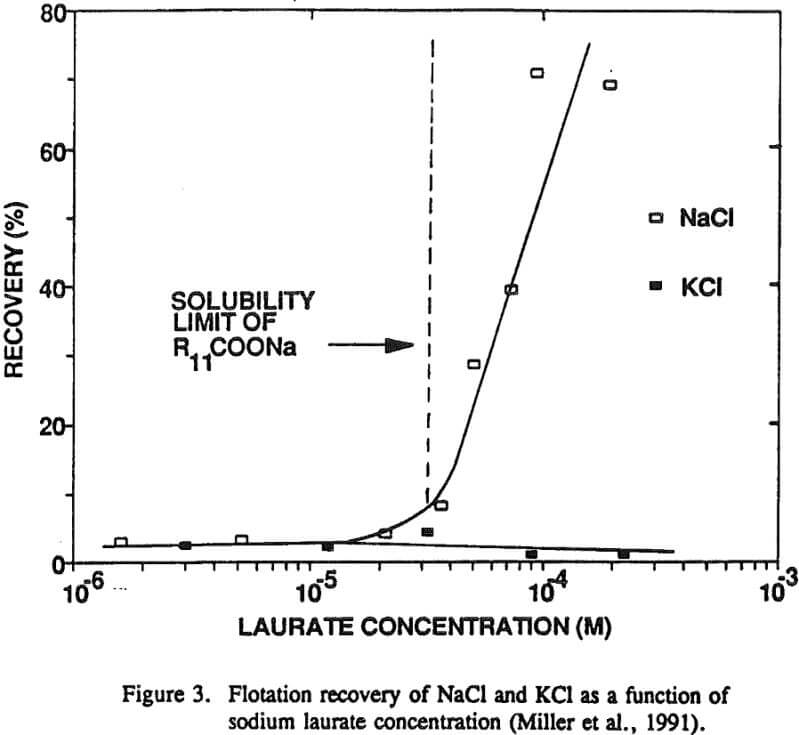
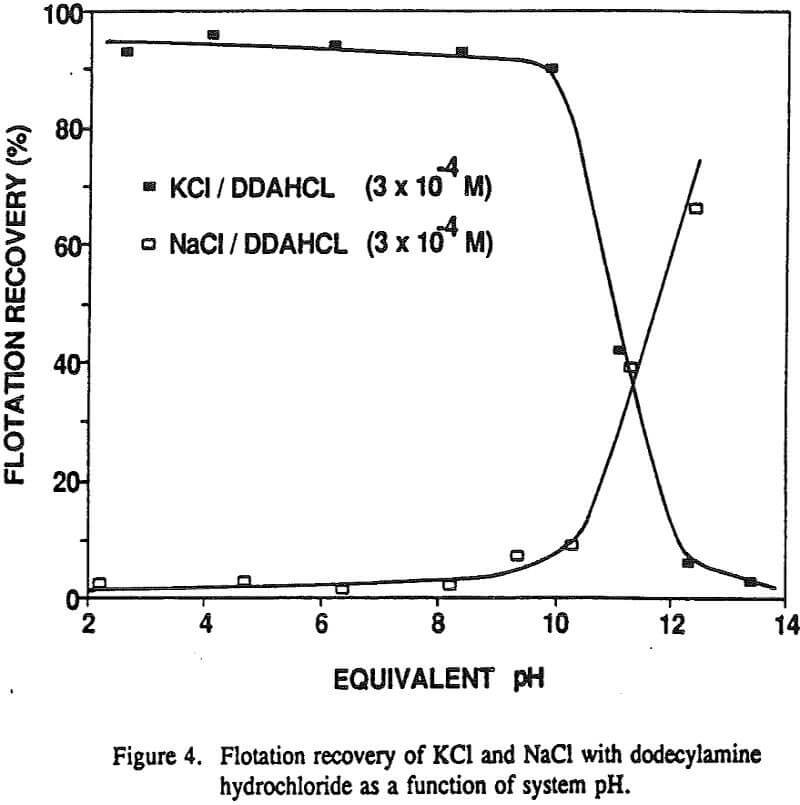
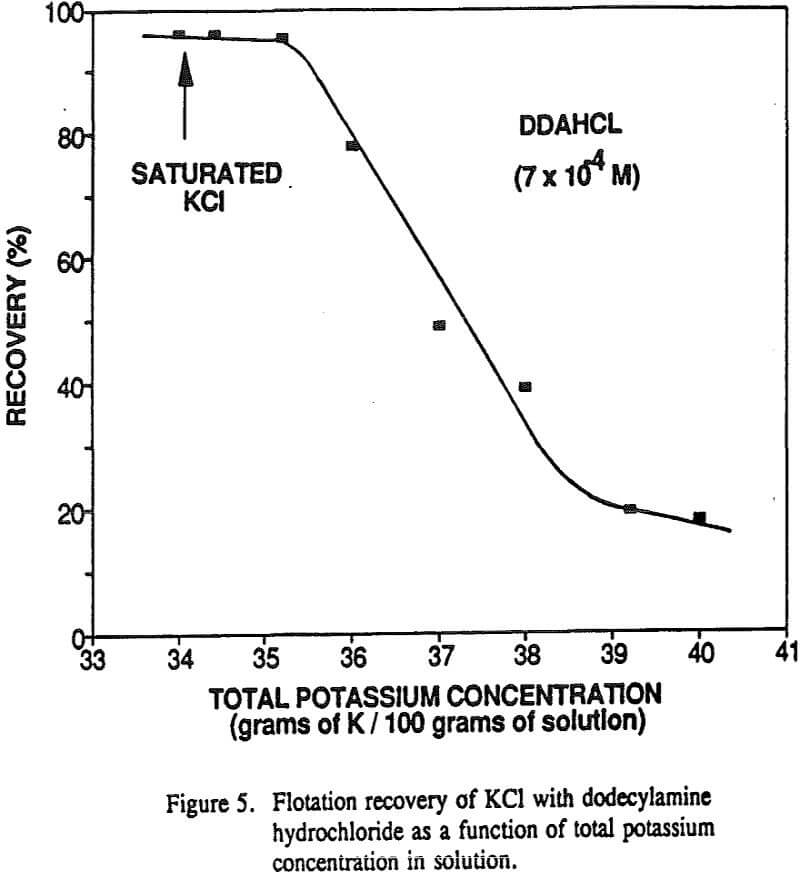
The nonequilibrium zeta potential results from the previous section allow for the sign of the surface charge to be predicted for alkali halides in their saturated brines. On this basis we can examine in more detail the hypothesis regarding the role of surface charge in the flotation of alkali halides. For example, the flotation response of KCl and NaCl salts was studied with dodecylamine hydrochloride and sodium laurate collectors.
Generally, the surface charge of the alkali halide salt should be fixed by the solution activity of the potential determining lattice ions. In this regard, KCl flotation by dodecylamine hydrochloride was examined at natural pH as a function of potassium ion concentration.
The preceding results and discussion clearly indicate that the adsorption of collector colloids and subsequent flotation of alkali halide salts are determined by coulombic forces associated with the surface charge of the collector colloid and the surface charge of the alkali halide mineral. In the following section, this hypothesis has been further tested for 21 different alkali halides based on bubble attachment studies.
It is also interesting to consider the flotation response of the NaI and NaI · 2H2O systems. It may be recalled that anhydrous NaI is negatively charged as predicted by the lattice ion hydration theory, whereas NaI·2H2O is positively charged. This example clearly highlights the significance of hydration in the charging process. When water is introduced into the NaI crystal lattice, the hydration of the Na+ in the lattice is substantial, in which case the iodide anion has a greater tendency to hydrate and leave the lattice surface site, thus resulting in a positive surface charge.