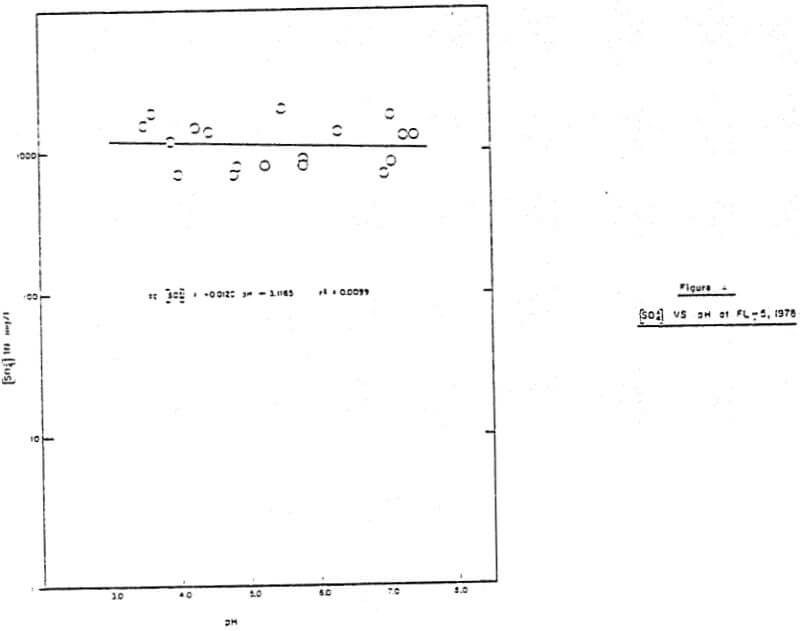
The first leaching step is the release of trace metals from the mineral phase due to sulfide oxidation. Sulfate was the ultimate reaction product in both the laboratory and the field, a result of the pH and oxidizing conditions which prevailed, and the relatively long reaction time. The rate of sulfide oxidation observed in batch reactors was a function of available sulfide surface area, dissolved oxygen concentration, and to a much lesser extent pH (equation 1). The dependence on surface area and dissolved oxygen indicates that the oxidation was a surface reaction with oxygen as the oxidizing agent. The influence of ferric iron as an oxidizing agent was minimal, as indicated by the extremely low aqueous iron concentrations (Figure 1, Table 2). The oxidation rate was also observed to be independent of pH at Minnamax (Figure 6).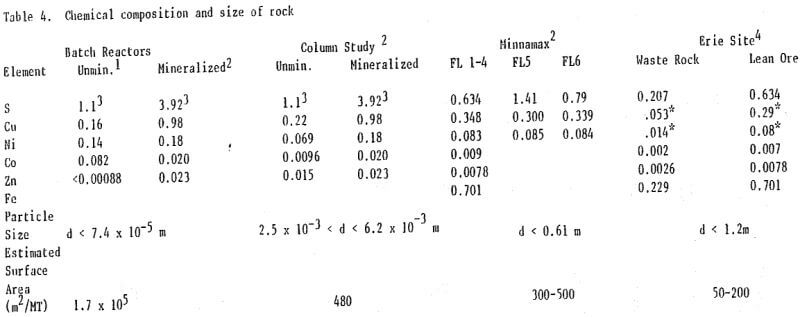
The variation of the sulfide oxidation rate with surface area, as indicated by percent S, was also observed in column experiments at Minnamax, and at Erie (Figure 4, Tables 2,6). An exception occurred at Erie where the sulfate release rate was greatest at Seep 1, a waste rock stockpile. This may have been due to the presence of anomalously high concentrations of Fe, Ni, Co, and Zn sulfides despite the copper content of less than 0.2%. This hypothesis is supported by the elevated concentrations of these metals in the Seep 1 leachate (Table 6).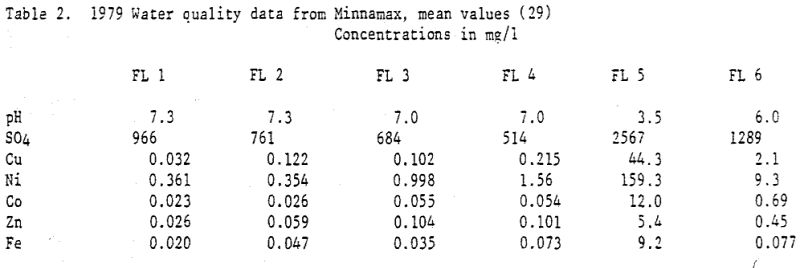
The order of observed concentrations of trace metals and sulfate was similar in both the laboratory studies and field studies. In all cases sulfate concentrations were the greatest by an order of magnitude and nickel concentrations were the highest of the trace metals. Aqueous iron and copper concentrations were much lower than those observed for nickel (Tables 2,5,6) despite the fact that their concentrations in the solid phase were several times as high (Table 4). This was most likely a result of the aqueous chemical behavior of the individual metals.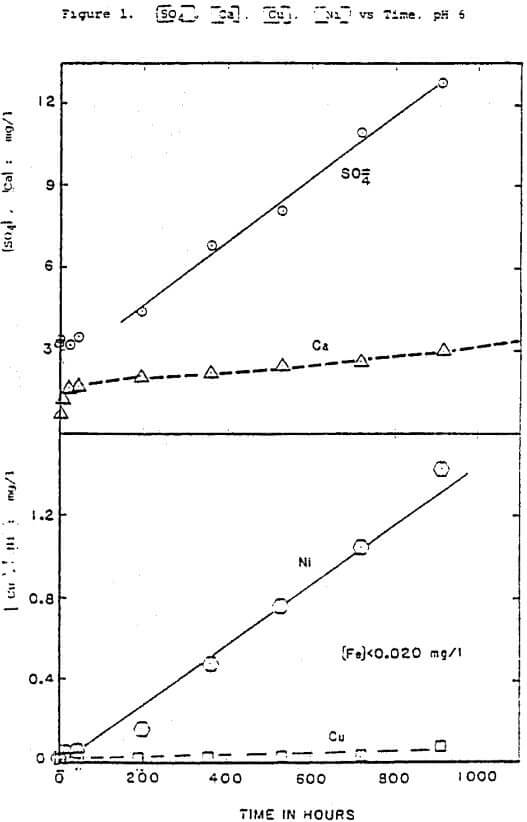
Secondary Reactions
The second step in the leaching of trace metals involves reactions which occur subsequent to their release from the sulfide mineral phase (secondary reactions). Iron and copper participate in reactions which result in their removal from solution, whereas nickel is more mobile and tends to stay in solution. The mechanism of iron removal under oxidizing conditions is most likely oxidation of ferrous to ferric iron with subsequent formation of y- FeOOH, lepidocrocite. This would result in an overall reaction stoichiometry as follows:
FeS(s) + 5/2 O2 + H2O → y-FeOOH(s) + 2H+ + SO4
Copper concentrations were most likely limited by the low solubility of copper. Copper removal may be due to malachite precipitation or adsorption onto silicates or other surfaces. Due to its low sulfide solubility log ksp = -36.1 (11) it also has a high tendency to participate in exchange reactions with metals of greater sulfide solubility. The high dependence of copper concentrations on pH further suggests that the race is limited by the low aqueous mobility of copper.

This is further supported by results from the computer program REDEQL2, which indicate that as pH increases above 5.6 the dominant form of copper is malachite (Figure 9). The predicted adsorption by silicate surfaces (lm²/l) also increased with increasing pH. Removal by adsorption onto solid oxides and exchange reactions with other metals in the sulfide form would also tend to increase with increasing pH, but these reactions were not considered in the program.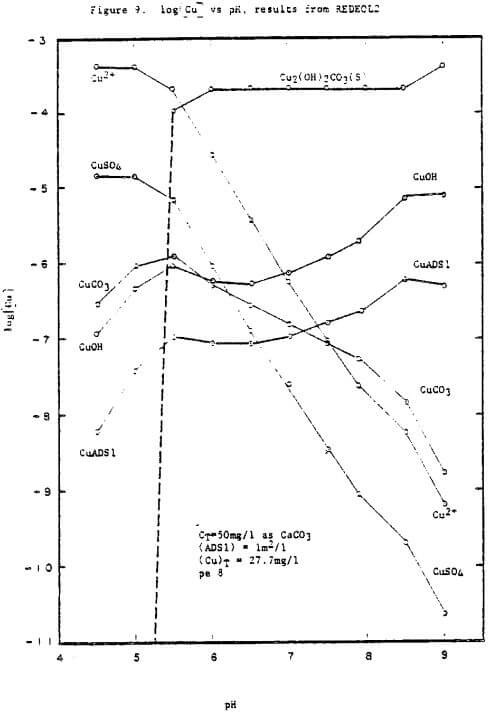
Below pH 8.5 the only nickel removal predicted is the result of adsorption onto the silicate surface; this removal increased with increasing pH (Figure 10). The magnitude of the removal predicted is quite small in comparison to laboratory and field observations. A larger surface area loading than 1 M²/l may more accurately represent the flow regime through a stockpile. Reactions not considered in the program may also contribute significantly to removal (e.g. adsorption onto oxides of iron or manganese, exchange reactions).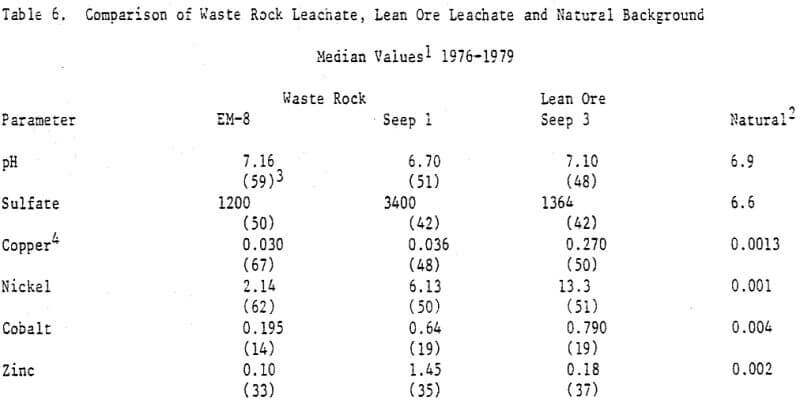
The ratio of trace metals to sulfate, the ordering of trace metal concentrations, and the dependence of trace metal concentrations on pH indicate that trace metal leaching rates are controlled by reactions subsequent to oxidation of sulfide minerals. If the majority of metals were transported (i.e. if leaching was controlled by the rate of sulfide oxidation) the sum of trace metal concentrations would approach 100% of the sulfate concentration, rather than the 0.3 to 1.7% observed at Erie. If secondary reactions did not remove metals from solution the aqueous metal concentrations would be in the same proportion as concentrations in the solid phase, assuming the oxidation rates of the various sulfide minerals were similar. However, aqueous nickel concentrations are much higher than copper and iron concentrations despite the comparatively low nickel concentrations in the gabbro. This is consistent with the predicted behavior of these metals in secondary reactions. Finally, trace metal concentrations increased with decreasing pH (Figure 7) while sulfate concentrations remained relatively constant (Figure 6). This indicates that the increase in trace metal concentrations was the result of increased aqueous mobility at the low pH values, rather than increased metal sulfide oxidation.