Table of Contents
Leaching is defined as a form of extraction in which a valuable constituent is removed from a solid body by mass transfer to a solution which, for the purpose of this discussion, is considered to be an aqueous medium.
Historically, leaching has been applied to the recovery and purification of many elements.
- The leaching of copper minerals, sulfides and oxides, by acid or by ammoniacal leach solutions with or without an oxidant.
- The leaching of gold and silver, either native metals or as -minerals by an oxygen-containing alkaline cyanide solution.
- The solubilization of certain uranium and vanadium minerals by oxygen or oxidant-bearing lixiviants.
Vat Leaching
Now, let us consider a representative submerged vat leaching system. Hundreds of thousands of tons of copper are produced each year by this method from oxide and mixed oxide-sulfide ores using sulfuric acid leach solutions. A typical submerged vat leaching plant is described as follows:
Ore is crushed to optimum size for copper extraction using two or three-stage crushing. This size will typically be from ¾ inch to 3/8-inch top size.
The crushed ore is then bedded in a vat with special care to prevent segregation of the coarse and fine fractions. Vats are typically 50 to 120-feet long, 50 to 100-feet wide, and 20 to 25-feet deep, and are arranged in rows with common walls between adjacent vats. Vats are usually constructed of monolithic concrete with an acid-resistant covering. This acid-resistant covering has continually proven to be a significant operational problem and a high cost item.
A leach solution typically containing 30 to 60 grams per liter sulfuric acid is used to cover the ore and is circulated with the pump to promote solution-ore contact. After a certain leach time, the leach solution is moved counter-current to the ore with the lowest grade copper solutions contacting the oldest ore in the leach cycle and the highest grade solution contacting the newest ore in the leach cycle. The highest grade solution in turn is advanced to the copper recovery system which can be either iron cementation, direct electrowinning, or solvent extraction followed by electrowinning .
Shortcomings of Vat Leaching
One thing which can be said about leaching that probably will not be argumentative to anyone in the hydrometallurgical field is, that the lixiviant solution must contact the copper-bearing mineral before leaching can occur. This sounds simple, but good solution-ore contact is difficult to achieve in a vat that measures on the order of 100-feet by 100-feet by 20-feet deep.
Picture, if you will, an undisturbed bed of ore as being the equivalent of 36,000,000 straws, 1/10-inch diameter by 20-feet long, standing on end and connected to the false bottom of a 100-foot by 100-foot vat. This spacing is roughly equivalent to 20 percent void space. Now, begin pumping 1,800 gallons per minute through these straws by upward percolation.
The use of downward percolation of leach solutions helps alleviate some of these problems, but not all of them. As an aid in understanding the hydraulics, consider what would happen to a water balloon resting suspended under water in a swimming pool. What would be the effect of gravity? Would the balloon rise, fall, or remain stationary? We believe it would remain stationary, as its weight would be exactly offset by its buoyancy.
The vat leaching operation at the Yerington Mine was started in October, 1953. The original plant facilities consisted of primary and secondary crushing, eight leaching vats, precipitation launders, sulfuric acid plant and the necessary maintenance, warehouse and administrative facilities.
The crushing plant is first used to fill the sulfide fine ore bin for the concentrator. The remaining time is used to fill the leaching vats. Normally, two leaching vats may be filled requiring about 20 hours. This is followed by 14 hours of crushing sulfide ore to fill the concentrator fine ore bin. In 1974, an average of 490,000 dry tons per month of oxide ore and 400,000 dry tons per month of sulfide ore were crushed.
For vat leaching, it is necessary to have a porous mass in the leaching vat for a free and even percolation of solutions. Considerable effort has been put into our agglomeration and bedding system for this purpose. There are eight chutes, one from each vibrating screen, that carry finished ore to the conveyor system leading to the leaching vats. After each chute is an adjustable water spray to moisten each layer of ore as it is deposited on the conveyor.
Oxide ore leaching is carried out in eight leaching vats. Each tank will hold about 12,700 dry tons of ore. They measure 120′ x 135′ x 20′ deep and are constructed of reinforced concrete. Originally, they were lined with 4″ of asphalt mastic, The top 1/3 of the asphalt mastic lining, because of deterioration, has been removed and replaced with concrete gunite. Each vat has a filter bottom 6 x 8 inch timbers, spaced on 16″ centers, were placed on the bottom.
A leaching cycle may be divided into four 17-19 hour leaching periods plus washing, excavating and bedding. There are normally five vats in the system, leaching or washing at one time. Acid is added to the solution flowing from the recirculation air lifts, as 93% H2SO4, during the recirculations. The acid is added in three additions as early as possible in the leaching cycle. The residual acid during the rest of the leaching cycle is enough to complete the leach.
Concrete launders are used to precipitate the copper from solution. Each launder measures 10′ x 58′ and is approximately 5′ deep. There is a gentle slope to the discharge end for easy cleaning. Each launder receives solution through three perforated 4-inch plastic hoses imbedded in the floor. The solution percolates upward through light gauge scrap iron and overflows an adjustable weir at the discharge end. The weir is adjusted upward as needed to keep fresh scrap iron in contact with the solution.


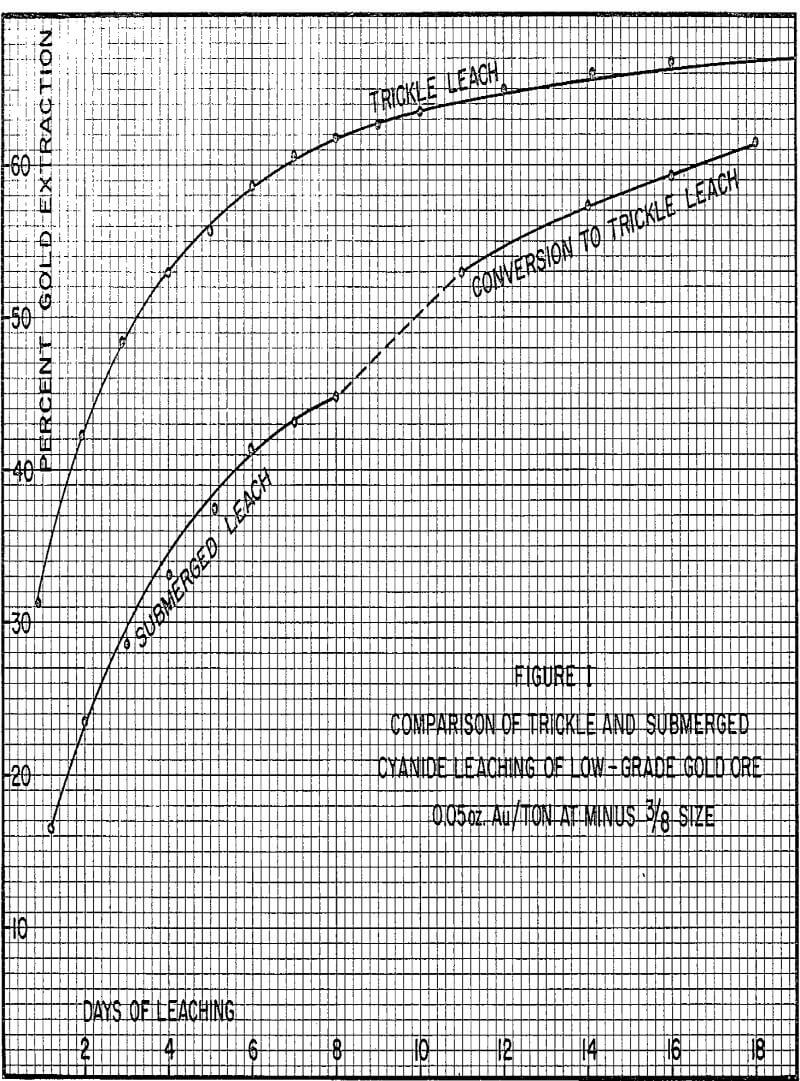
Trickle Leaching
With this leach system, the ore in the vat would not be submerged but would be drained at all times allowing gravity to carry the trickle solutions past each individual ore particle. This solution would be collected at the bottom of the vat and sent to solvent extraction to produce a high purity concentrated copper solution for electrowinning.
Several positive effects are produced by the change to trickle leaching. First of all, slowly trickling the solution through the ore vat will allow gravity to draw the solution more evenly through the ore. Since the ore is not flooded with solution, the carbon dioxide generated within the vat of ore can readily escape without forming channels. The low flow rates employed would not tend to enlarge any natural channels that did exist.
It is estimated that an average raffinate from solvent extraction would contain 0.3 grams per liter copper or less, and about 6.0 grams per liter sulfuric acid, which on a typical ore would produce an average feed to solvent extraction of 3.0 grams per liter copper with less than 1.0 gram per liter sulfuric acid. This type of reduction in acid content is made possible because of the greatly reduced copper content of the leach solution. This improves the kinetics of the leach reaction, as was described earlier.
With trickle leaching, each vat of ore could be operated as an independent leach system able to take into account variations in ore grade and leaching kinetics. Therefore, a trickle leach system would reflect all the way back to mine planning, and could produce considerable savings by allowing more freedom in production scheduling than is possible in a balanced submerged vat leaching system. With a trickle leach system, the time of leach, solution flow rate, and acid strength could be adjusted for higher or lower-grade ores.
The open interstices between ore particles in trickle leaching provide a conduit for the escape of gaseous reaction products. This escape route precludes the build-up of pressure pockets which eventually gain sufficient buoyancy to form “eruptions” in flooded vats. These eruptions form channels of low flow resistance which decrease solution contact with ore particles and thereby lower recovery.