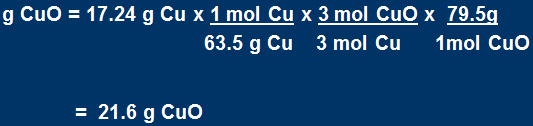In this Example of Stoichiometry of Excess Reagent Quantities determination we show how do you determine how much of the excess reagent is left over & how to calculate how much more of the limiting reagent is needed to use up the excess reactant?

Introduction: So far we have assumed that a given reactant is completely used up during the reaction.
In reality
reactions are often carried out in such a way that one or more of the second reactants actually are present in EXCESS amounts.
Definitions
EXCESS REACTANT = the reactant in excess
LIMITING REACTANT = the reactant that completely reacts
THE LIMITING REACTANT determines the yield of the product (how much product(s) will form)
A Simple Analogy

Imagine you work at McDonalds™
You have 10 hamburger buns and 5 beef patties
How many regular hamburgers can you make?
Da Answer
Indeed, you would get 5 regular hamburgers!
And what was left over?

Connecting the Lingo
There would be 5 hamburger buns in EXCESS!
Therefore, the beef patty is known as the LIMITING ingredient since it “limits” or determines how many regular buns can be made!
Note that

we do not predict based on the number of hamburger buns
Example 1
If 20.0 g of hydrogen gas react with 100.0 g of oxygen, which reactant is present in excess and by how many grams?

Step 1
The balanced equation:

Step 2

First PREDICT which reactant is limiting (it’s ok if you predict wrong)
USUALLY the reactant with the least number of moles is limiting (but not always)
Convert masses to moles
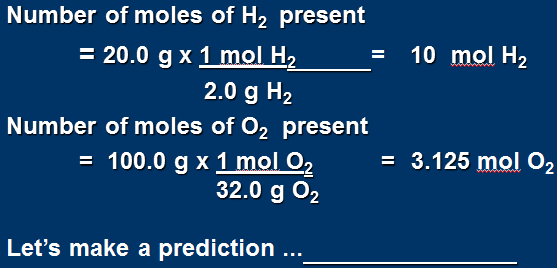
Prediction: O2 is limiting
Mass of H2 that reacts with 100.0 g O2
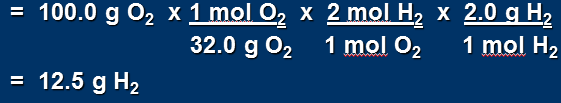
Analysing the numbers
What we have: 100.0 g O2 and 20.0 g H2
We predict O2 is limiting (i.e. all 100.0 g reacted)
We calculated that we would need 12.5 g H2
Is the prediction correct ?

Da Answer (again!)
Yes!! Prediction is correct
only 12.5 g H2 is required, so we have an excess of 7.5 g H2 (20.0 g – 12.5 g)
so H2 is in EXCESS of 7.5 g.
The other side of the coin
So what if we predicted that H2 was limiting?
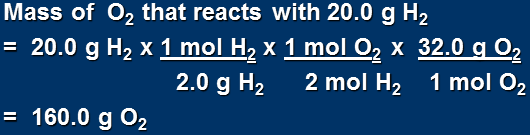
Therefore
If ALL 20.0 g of H2 were to completely react we would need 160.0 g of O2
BUT we only have 100.0 g of O2
So the prediction that H2 limiting is INCORRECT!
Example 2.

If 79.1 g of Zn reacts with 1.05 L of 2.00 M HCl,
a) Which reactant is in excess and by how much?
b) What is the mass of each product?
a) which reactant is excess?
The balanced equation:
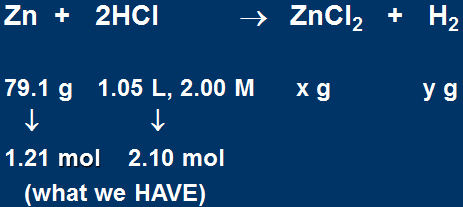
Prediction: Zn is limiting
Moles of HCl required

Therefore 2.42 mol HCl would be required to react with 1.21 mol Zn.
We ONLY have 2.10 mol HCl
So is our prediction correct?
Uh Oh! You’re wrong!
We would need more HCl (2.42 mol) than what we have (2.10 mol) if all the Zn were to react
Thus: Zn is in excess, and HCl is limiting!
To find how much in excess:

We must find how many moles of Zn is required to react with 2.10 molHCl Mol of Zn
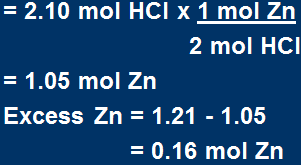
b) mass of products?
Since HCl is limiting we MUST use this amount to calculate the mass of products
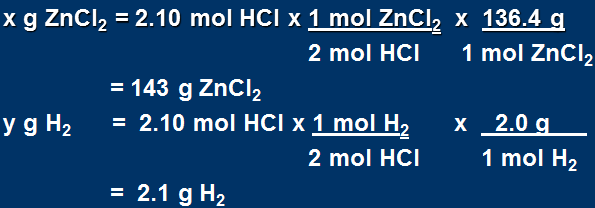
Example 3:
3.00 L of 0.1 M NaCl reacts with 2.50 L of 0.125 M AgNO3. Calculate the yield of solid AgCl (in grams) that will be produced.
This problem requires us to determine how much product (AgCl) will form, so we will need to first determine which reactant is limiting.
The balanced equation:
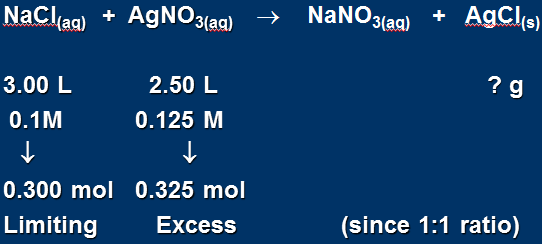
NaCl limiting
Therefore: mol NaCl = mol AgCl = 0.300 mol (also 1:1 ratio)
Mass of AgCl = 0.300 mol AgCl x 143.5 g AgCl/1 mol AgCl = 43.1 g AgCl
Percent Yield

Often 100% of the expected amount of product cannot be obtained from a reaction
The term “Percent Yield” is used to describe the amount of product actually obtained as a percentage of the expected amount
Reasons for reduced yields
A) the reactants may not all react because:
i) not all of the pure material actually reacts
ii) the reactants may be impure
B) Some of the products are lost during procedures such as solvent extraction, filtration etc

The equation:
Percent Yield = ACTUAL YIELD/THEORETICAL YIELD x 100%
Actual yield = amount of product obtained (determined experimentally)
Theoretical yield = amount of product expected (determined from calculations based on the stoichiometry of the reaction)
The amounts may be expressed in g, mol, molecules
Types of calculations
A) Find the percentage yield, given the mass of reactant used and mass of product formed
B) Find the mass of product formed, given the mass of reactant used and the percentage yield
C) Find the mass of reactant used, given the mass of product formed and percentage yield
Note that the percentage yield must be less than 100%
But when calculating the theoretical yield assume a 100% yield
Example 1
When 15.0 g of CH4 is reacted with an excess of Cl2 according to the reaction:
CH4 + Cl2 → CH3Cl + HCl
a total of 29.7 g of CH3Cl is formed. Calculate the percentage yield.
The solution
The actual yield of CH3Cl = 29.7 g
To find the theoretical yield of CH3Cl: (assuming a 100% yield)

Then:
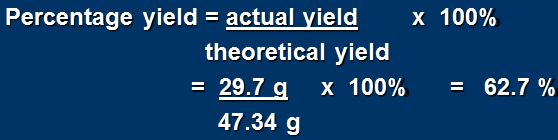
Example deux!

What mass of K2CO3 is produced when 1.50 g of KO2 is reacted with an excess of CO2 if the reaction
has a 76.0% yield? The reaction is:
![]()
The solution:
We are looking for the actual yield (some idiot forgot to weigh and record the mass of product!)
First calculate the mass of K2CO3 produced (assuming a 100% yield) i.e. the theoretical yield
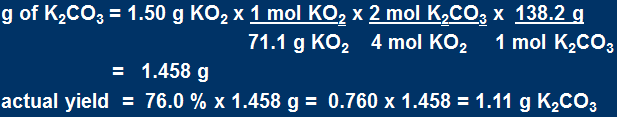
Last (but not least) example
What mass of CuO is required to make 10.0 g of Cu according to the reaction
2NH3 + 3CuO → N2 + 3Cu + 3H2O
if the reaction has 58.0 % yield?
Here we go again
Actual yield = 10.0 g Cu
From the percentage yield equation, calculate the theoretical yield of Cu.
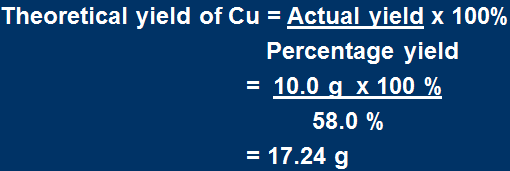
Now find the mass of CuO:
Use this theoretical yield and find the mass of CuO that would be needed: