Table of Contents
In the processing of numerous types of minerals to recover their values, enormous tonnages of gangue materials are produced and discarded to storage areas near the processing plants. In this country, primary copper producers alone produce 125 million tons of tailings per year. Zinc, lead, asbestos, and roofing granule operations also produce large tonnages of mineral wastes. The vast accumulation of these wastes from past and current operations are generally unattractive and may contribute to air and water pollution.
To help cope with mineral waste accumulations, the Federal Bureau of Mines is engaged in a broad investigative program to develop practical methods for stabilizing and utilizing numerous wastes. Because of the enormous tonnages of tailings accumulated from past operations, major emphasis is directed to studying stabilizing techniques. However, since the solid mineral wastes are generally discharged as finely ground materials composed chiefly of quartz, feldspar, serpentine, or chlorite, an investigation was conducted to determine if this waste could be utilized as a raw material for producing steam-cured building bricks. Utilization of these material wastes could eliminate the need for excavating new pits for brick raw material production and thus prevent the spoiling of the countryside and simultaneously avoid the creation of fine dust, a potential air pollutant.
Bricks composed of sand and lime, hardened by curing in high-pressure steam with formation of a hydrated calcium silicate bond, were first made commercially in Germany about 1898. The application of this process varied widely from one country to another, depending upon a number of factors. The dominant factor was the availability of suitable sand, clay, and concrete aggregate. In parts of Germany, calcium silicate bricks are widely used as internal and external structural building materials, and in Holland and U.S.S.R., this type of brick is now used in great quantities. On the other hand, usage in the United States is limited because the consumer prefers the use of concrete block. In general, calcium silicate bonded bricks compare favorably with the competitive products, although each product and method of production has its relative advantages and disadvantages.
Several research groups have studies the possibility of producing ceramic structural products from a variety of solid mineral wastes. West Virginia University, under sponsorship of the U.S. Department of the Interior’s Office of Coal Research, developed a process for making building bricks and other structural units from fly ash. Stanford University, under a Federal Bureau of Mines grant, developed a process for producing calcium silicate bonded bricks from California gold mine tailings, and the Colorado School of Mines, also working under a Bureau grant, investigated the manufacture of building bricks by the extrusion and steam-curing method from various mine and mill waste accumulations located throughout Colorado. Building bricks were made by Bureau of Mines researchers by the dry press method from copper mill tailings.
Although the solid mineral wastes, if convertible to building bricks, represent an abundant and cheap raw material, most of these wastes are at considerable distances from population centers where the principal markets are located. At present, the manufacture and sale of building bricks depend on local raw materials and nearby markets. Therefore, a large-scale, steam- curing, brickmaking operation using solid mineral wastes would have to produce quality brick at a low enough cost to cover shipping charges.
Brick Specifications and Manufacturing Methods
There are no established specifications for raw materials for brickmaking, only for the finished bricks. In general, suitability is determined by tests that simulate the brickmaking process utilized. Sand-lime building brick are designated grades SW and MW. According to ASTM specification C73-67, grade SW “is intended for use where exposed to temperatures below freezing in the presence of moisture,” and grade MW “is intended for use where exposed to temperatures below freezing but unlikely to be saturated with water.” Physical requirements for grades SW and MW are given in table 1.
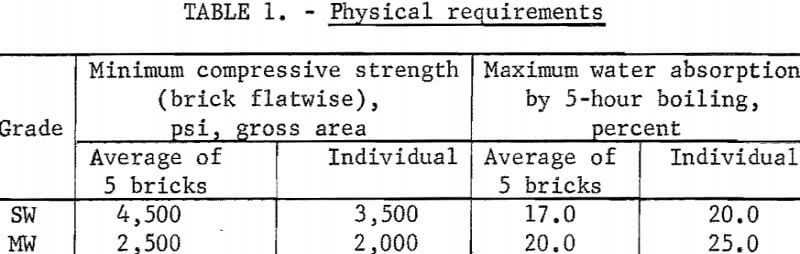
Facing brick must meet grade SW or MW standards and are divided into three types—FBA, FBS, and FBX. The FBA designation covers brick used to obtain special architectural effects; type FBS is for general use in exposed exterior and interior masonry walls; and type FBX is for general use in exposed exterior and interior masonry walls and partitions where a high degree of mechanical perfection, narrow color range, and minimum permissible variation in size are required. Because the mineral wastes vary in content of possible coloring minerals, considerable latitude in color is offered for production of facing brick.
Solid Mineral Wastes
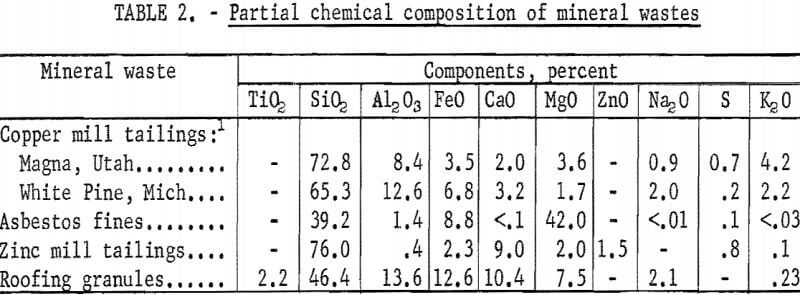
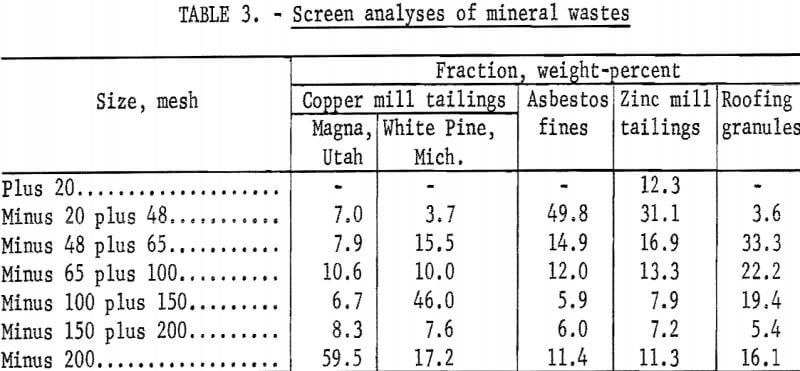
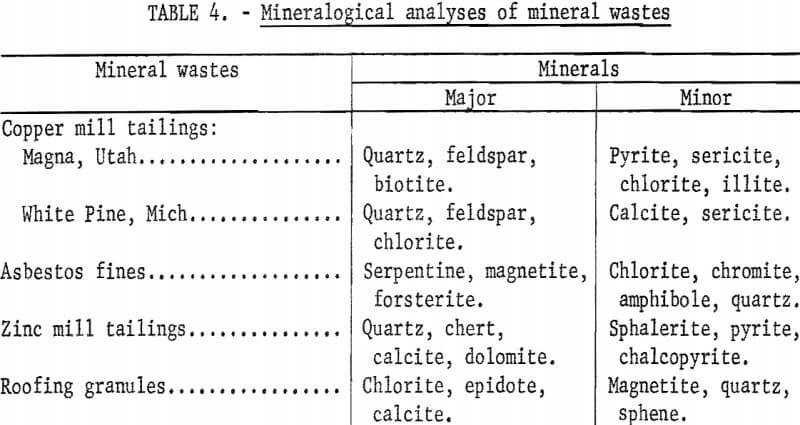
To demonstrate the versatility of the process, samples of mineral wastes representative of the types of wastes being discarded by mineral processing plants throughout the country were obtained for testing. Samples tested were copper mill tailings from Kennecott Copper Corp., Magna, Utah, and White Pine Copper Co., White Pine, Mich.; roofing granules from General Aniline and Film Corp., Blue Ridge Summit, Pa.; asbestos fines from Ruberoid Co,, Hyde Park, Vt. ; and zinc mill tailings from Eagle-Picher Industries, Inc., Miami, Okla. These samples were dried and divided into representative head samples for chemical, particle size, and mineralogical analysis. The results are given in tables 2-4.
Laboratory Test Procedures
The standard procedure for producing steam-cured building bricks from the solid wastes used in this study comprised drying, screening, mixing with bonding additives, adding water, forming, curing, and cooling. However, as the wastes are all chemically different, slight procedural variations were required with each sample in order to produce a suitable product. These variations are pointed out in the following sections that describe the testing of the mineral wastes. A general description of the process follows.
The wastes were dried in an oven at 110° C and screened through 20 mesh to break up lumps. To facilitate mixing and to attain a homogeneous mixture, the additives, either Ca(OH)2 or portland cement type I, were screened through 20 mesh before being blended with the tailings. The additives, used in amounts of 7 or 10 weight-percent, increased green strength during forming and promoted bonding during curing. Mixing was carried out for 15 minutes in a 1-gallon glass container. The blend was placed into a bowl and prepared for pressing by slowly adding water and handmixing to prevent lumping. The amount of water depended upon the type of additive being used. When Ca(OH)2 was used, 5 weight-percent water was sufficient, but with cement, the amount of water added was such that the ratio of water to cement was 0.5. In either case the amount of water used was sufficient to cause the mixture to stick together when compressed by hand. Cylindrical specimens, 2 inches in diameter and about 1-½ inches thick, were pressed at either 4,000 or 6,000 psi on a 12-ton-capacity Carver laboratory press.
Steam curing was conducted at 194° C and 200 psig for periods of 4 to 6 hours in a CENCO autoclave equipped with an automatic temperature control device. On completion of the curing cycle, the specimens were ovendried until ready for testing in accordance with ASTM designation C67-66.
Preliminary Testing
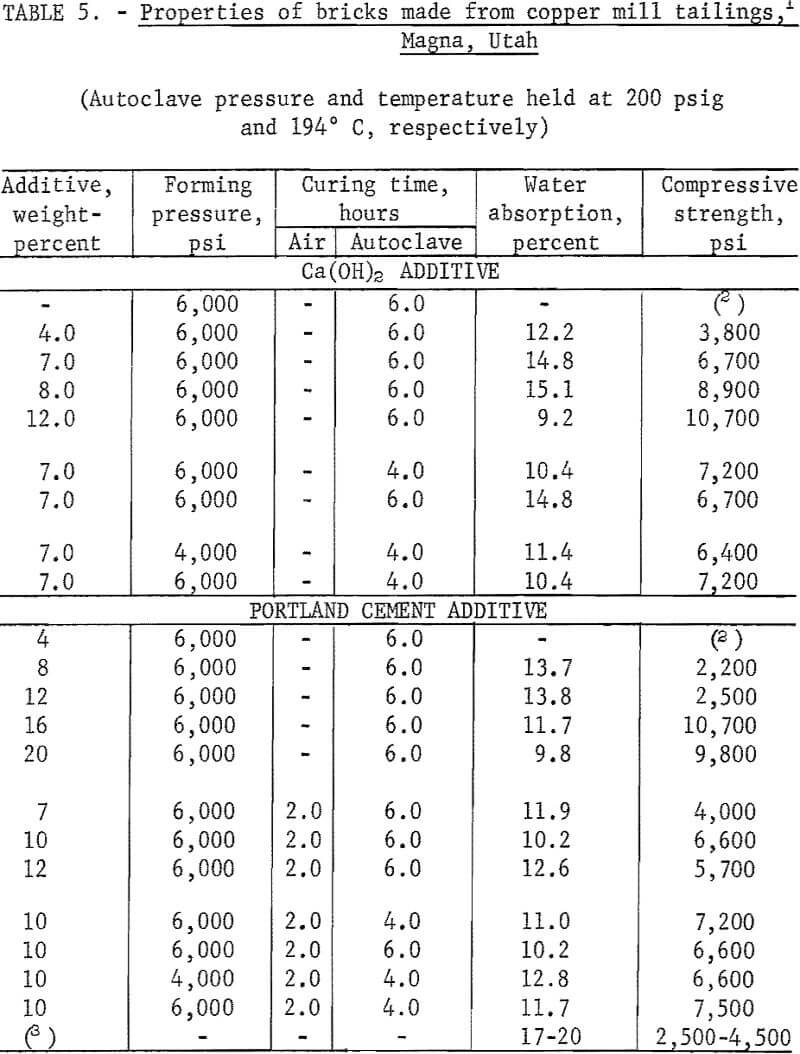
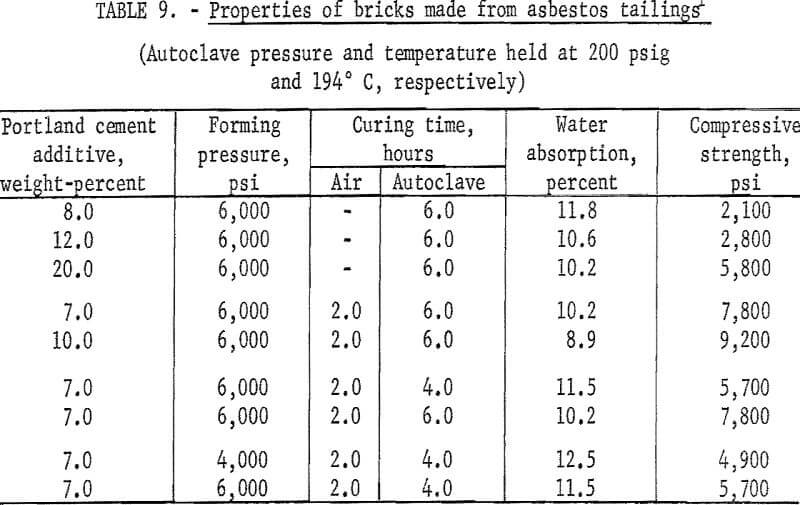
The factors influencing the strength of sand-lime bricks were defined and evaluated in preliminary tests utilizing copper tailings from Kennecott Copper Corp., Magna, Utah. Initial testing encompassed screening the tailings through 20 mesh, adding either Ca(OH)2 or portland cement type I as binder, moistening with water, dry pressing at 6,000 psi into 2-inch-diameter by 1-½-inch-thick specimens, and autoclaving for 6 hours at 200 psig. A series of comparative tests was run using as-received tailings without additives. The tests proved that (1) bonding did not occur without the presence of a binder, (2) forming at 6,000 psi and curing at 200 psig with either binder produced a strongly bonded product, (3) bonding with cement required the specimens to be precured at room conditions for 2 hours before autoclaving. Further evaluation of the critical fabrication or processing parameters, except for temperature and pressure which were held constant at 194° C and 200 psig, respectively, established the following conditions for producing brick from the copper mill tailings: Binder Ca(OH)2 7.0 percent, water 5 weight-percent, forming pressure 4,000 psi, and autoclaving time 4 hours. If portland cement is used as the binder, then the following conditions apply: Binder 10 percent with enough water added to maintain the ratio of water to cement constant at 0.5, forming pressure 4,000 psi, precuring time at room conditions 2 hours, and autoclave 4 hours. For both conditions, the autoclaving period of 4 hours does not include heating or cooling time, which amounts to about 1 hour for each. The results of these tests are presented in table 5.
Testing of Other Wastes
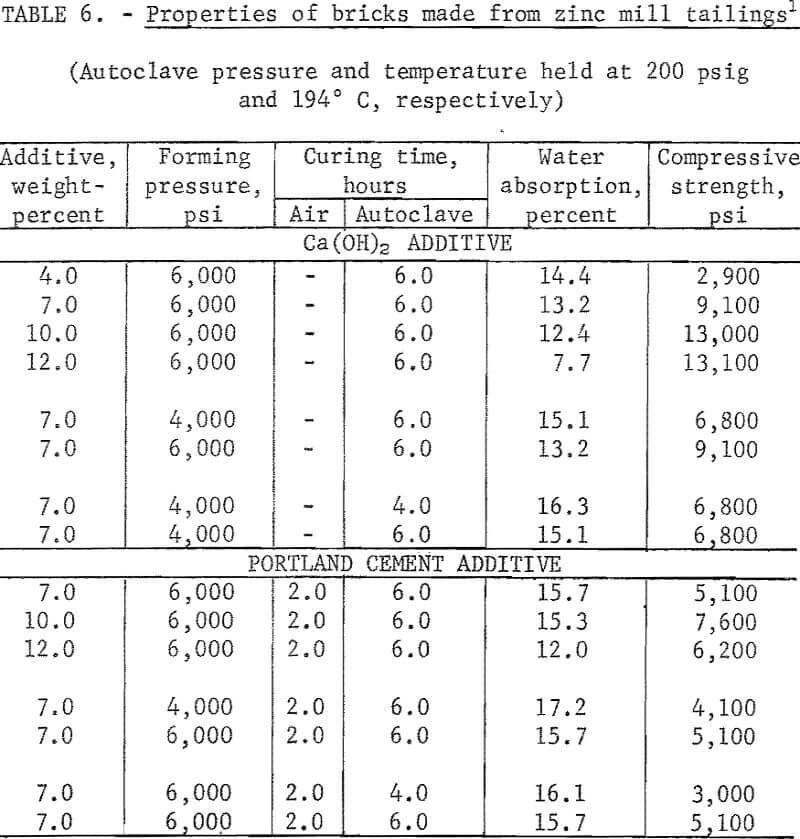
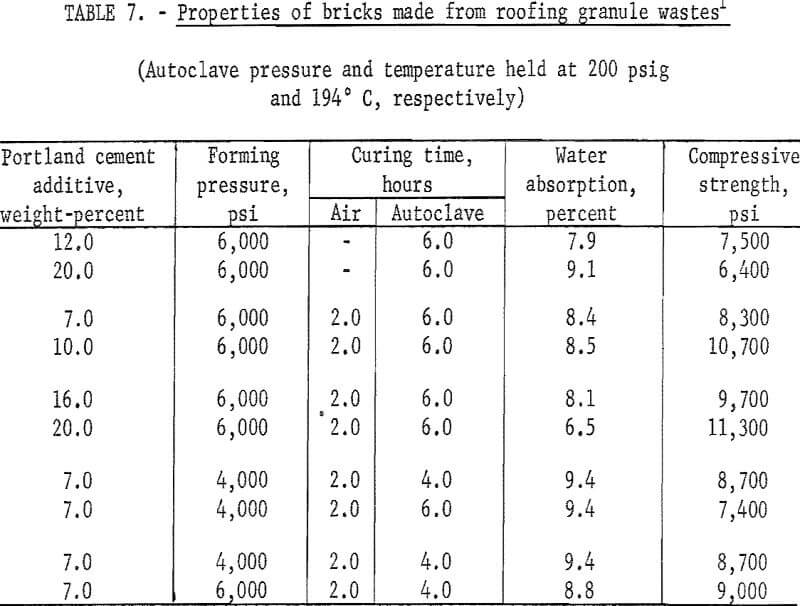
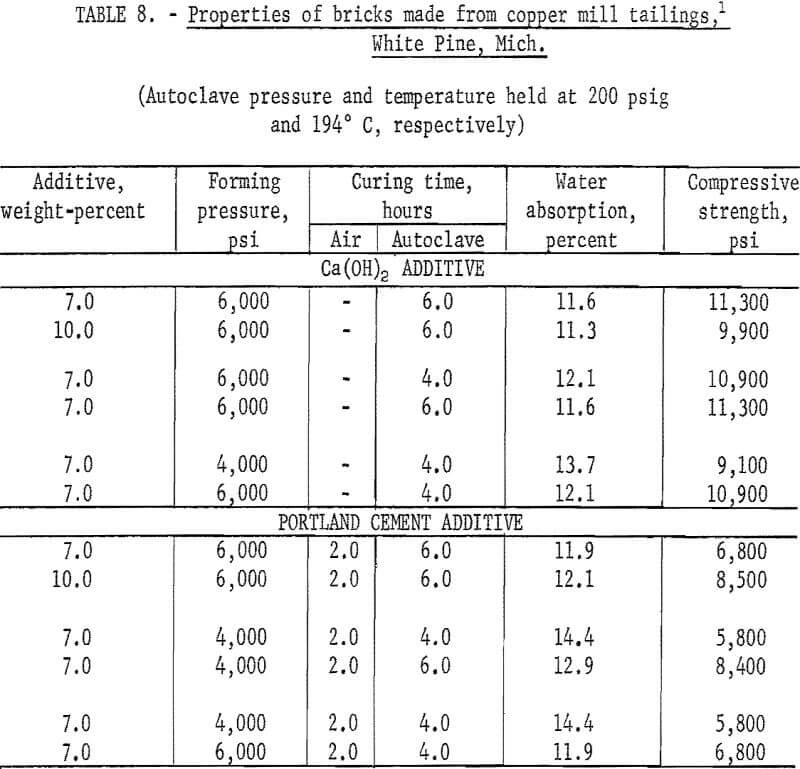
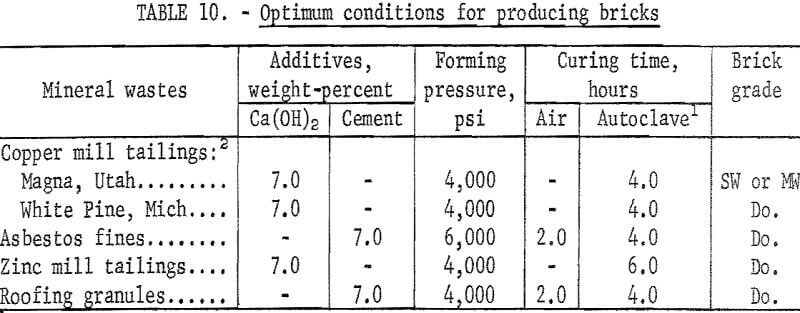
Experiments were conducted to determine if other mineral wastes could also be used for making building bricks by the same method developed for the copper mill tailings. With each waste, a series of tests was made to determine the effects of types and amounts of binder added, forming pressures, precuring times, and autoclaving times. In each case the wastes were screened through minus 20 mesh; binding additives Ca(OH)2 or portland cement were added in varying concentrations; forming pressures were varied between 4,000 and 6,000 psi; autoclaving time, between 4 and 6 hours; and all the specimens were autoclaved at 200 psig. As the data presented in tables 6-9 indicate, building bricks could be produced from all of the wastes tested. Linear shrinkages of all specimens produced were zero, and bulk densities ranged within the normal range of 105 to 150 pounds per cubic foot as required for this type of brick. A summary of the optimum conditions for producing brick from each of the wastes tested is presented in table 10.
Economics of Brick Production and Marketing
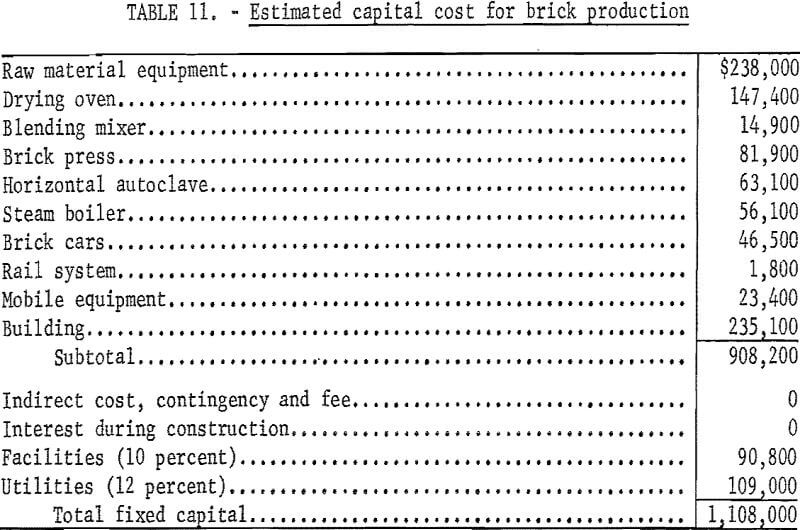
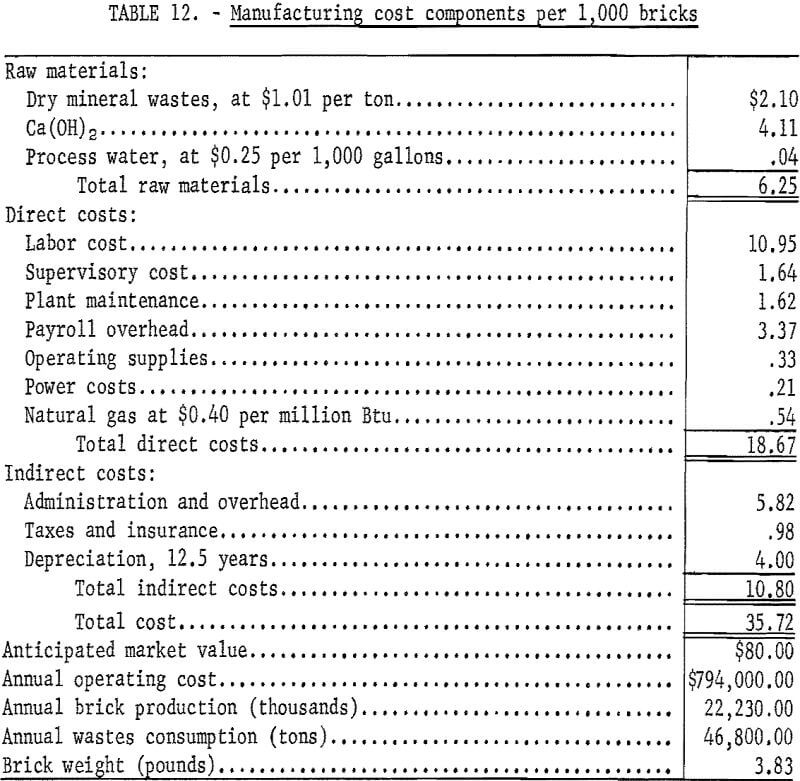
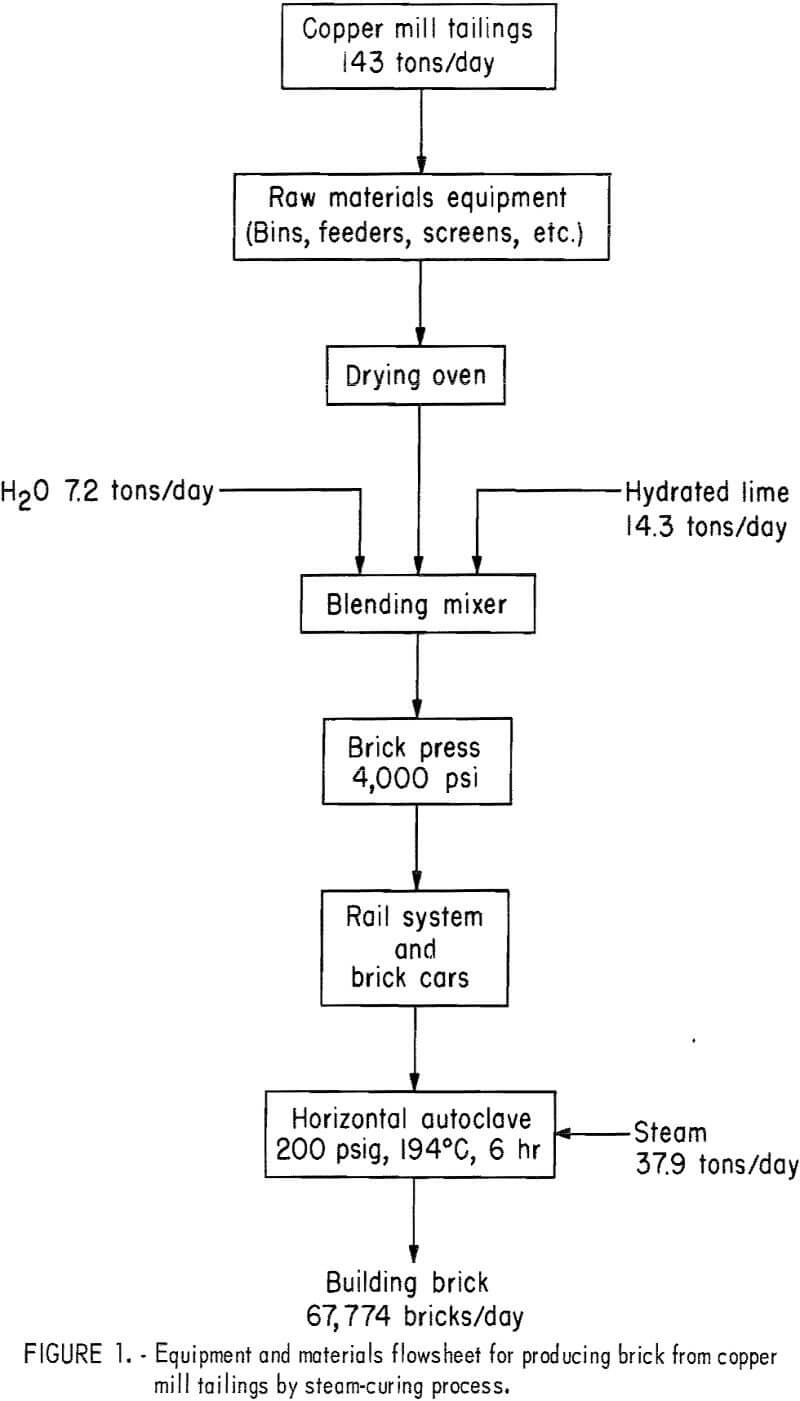
An economic evaluation of the process was prepared based on the production of 67,774 bricks per day using 143 tons of copper mill tailings. An equipment and materials flowsheet is presented in figure 1. A 24-hour day, 7-day week with a scheduled annual downtime of 10 percent is used in the evaluation; equipment costs are for mid-1971 (Marshall and Steven’s Index 321.0), and depreciation is assumed to be linear over a 12.5-year life. Estimated power and natural gas costs are 1 cent per kilowatt-hour and 40 cents per 1,000 cubic feet, respectively. Boiler water cost for the process is 25 cents per 1,000 gallons, and a labor rate of $3.90 per hour is assumed for the operation. The total annual fixed capital cost is shown in table 11, and the estimated cost of $35.72 for 1,000 bricks is shown in table 12. The cost of shipping bricks from Salt Lake City, Utah, to Denver, Colo., or San Francisco, Calif., would be $28 and $29, respectively, per 1,000 bricks, based on quoted rates of $0.73 and $0.76 per hundredweight, respectively. Anticipated market value is about $80 per 1,000 bricks. Equivalent rates for shipment of tailings are not on current schedules but might be about half the cost of shipping brick if manufacturing were to be considered at destination.
Conclusion
This investigation proved that various types of industrial mineral wastes can be economically used as raw materials for producing building bricks by the steam-curing process. Commercial acceptance of this process would aid in conserving the Nation’s natural resources and also would alleviate the pollution problems created by the mismanagement of mineral solid wastes.
