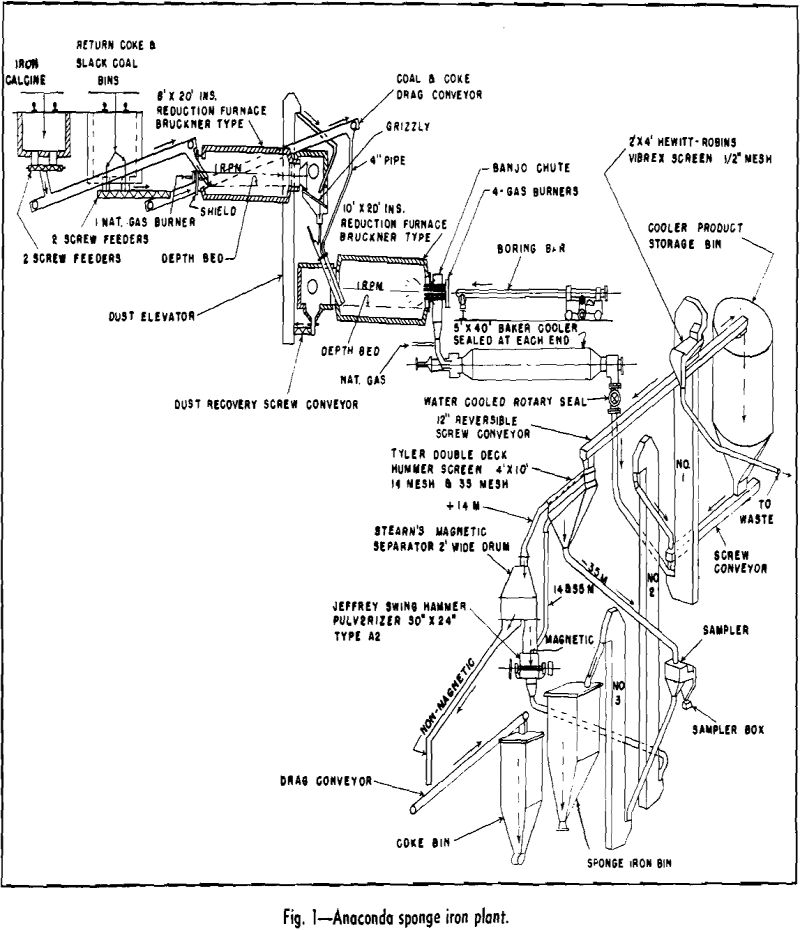
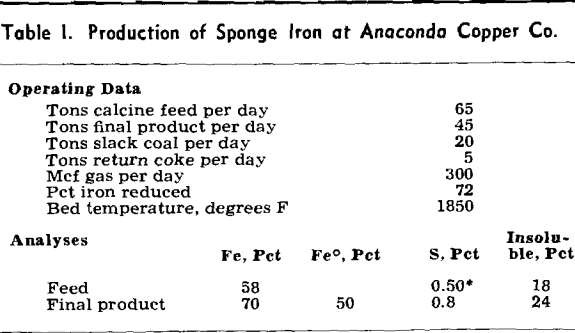
Sponge iron as produced at Anaconda is a fine, —35 mesh, impure product, about 50 pct metallic iron, obtained from the reduction of iron calcine at a temperature of 1850°F by use of coke resulting from slack coal. The metallic iron particles are bulky and spongey and precipitate copper readily and rapidly from a copper sulphate solution.
A good deal of work has been done in the last 50 years on the production of sponge iron. The objective in some cases has been the production of a precipitant for copper from solution, but the bulk of the work has been done for the production of open-hearth steel furnace stock. The production of an open-hearth stock presents two problems rather than one: first, production of the sponge iron, and second, what is perhaps of equal difficulty and importance, conversion of the sponge iron into a form suitable for use in the open-hearth furnace. So far as is known to the writer, none of the sponge iron processes tried in the past have proved to be economically feasible.
All indications are that the actual reduction of the iron is accomplished by carbon monoxide below the surface of the bed, which is 30 in. deep at its center. Apparently there is a constant interchange:
Fe2O3 + 3CO = 2Fe + 3CO2 CO2 + C = 2CO
Actually iron oxide is reduced by CO at somewhat lower temperature than the 1850°F used in the process, but this temperature is necessary to obtain a satisfactory rate of furnace production.