This paper reviews several new applications of solvent extraction developed in conjunction with a research project carried out under the AEC Raw Materials Division by the Dow Western Division laboratories during the last two years.
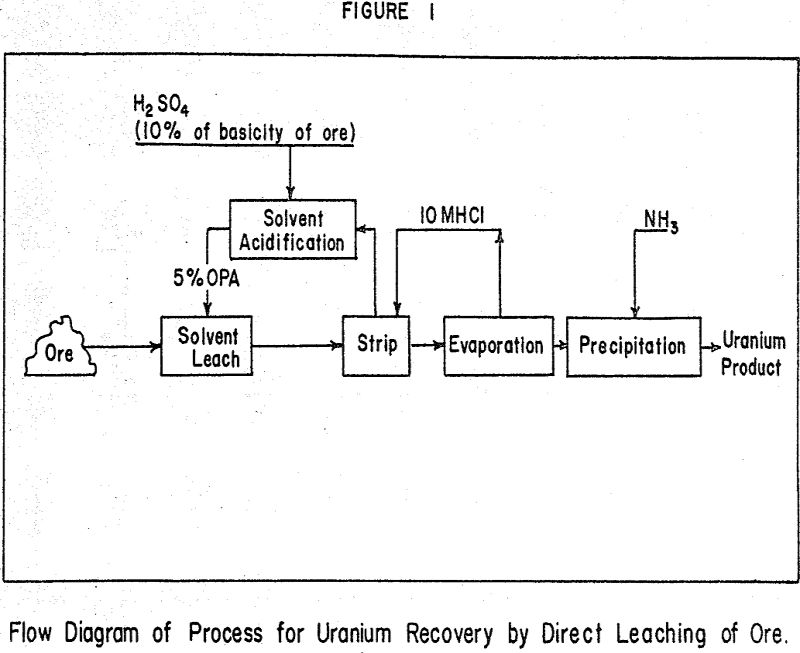
Several methods for direct solvent leaching of ores have been investigated by AEC contractors. The method studied at this laboratory is the only one that offers lower acid consumption than an aqueous leach. Most recovery processes now in use are applied to clarified aqueous liquors obtained by leaching with acid, sand-slime separation, thickening and filtration. A savings in operating costs is evident if these aqueous treatment steps can be eliminated.
The extractant is a commercially-available mixture of mono and dioctyl esters of phosphoric acid (OPA) diluted with a volatile solvent such as methyl ethyl ketone. It is essential that some mineral acid be added either to the solvent before contact with the ore or to the slurry of organic extractant and ore. Sulfuric acid was found to be superior to other mineral acids for this use.
Alkyl amine extractants cannot be used in the presence of even small amounts of solids because stable emulsions result. Alkyl phosphate extractants can be used to extract dilute slurries with little entrainment but as the slurry density increases the entrainment increases also.
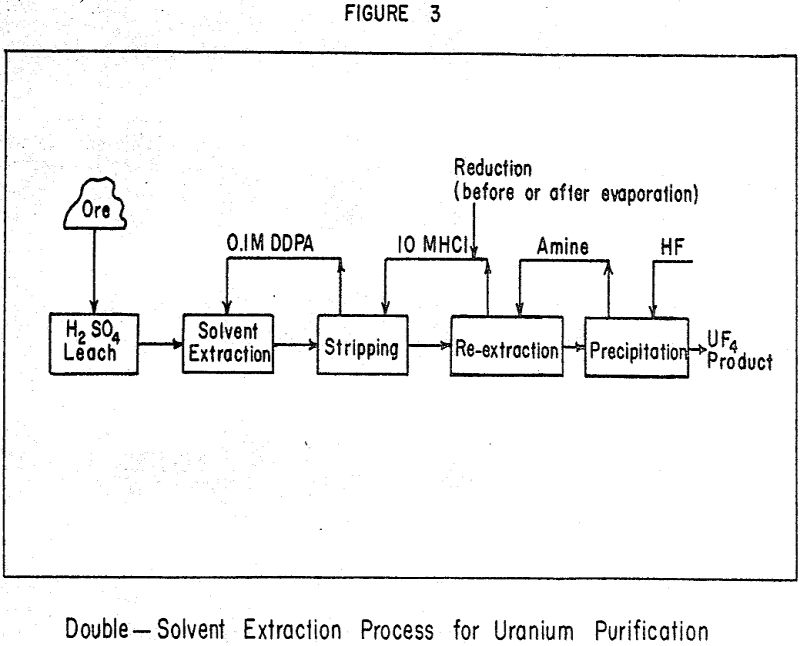
The current processes for the preparation of purified uranium salts are quite costly because of the large number of processing operations and the separation of concentration and purification into two distinct, widely-separated facilities. It seems evident that the costs could be reduced considerably by producing the final purified product directly at the ore-treatment plant, substituting cheaper acids for the rather expensive nitric acid used in the purification and precipitating the purified UF4 product directly from the final strip solution.
The first solvent extraction step is similar to that now used in several uranium mills on the Colorado Plateau. Either a mono- or a dialkyl phosphate in kerosene is used to extract a uranyl sulfate complex from the sulfuric acid leach liquor. The uranium is stripped from the solvent phase by use of strong hydrochloric acid and is reduced in the acid to the uranous state. Electrolytic reduction appears to be efficient and economical. It is then re-extracted as a chloride anion complex by a solvent which is selective for uranium in the reduced state. Various long-chain, secondary amines have been found to be quite selective. The uranium is stripped with water and precipitated by addition of HF. The product is filtered and dried to yield very high-purity uranium tetrafluoride.