Some of the more important technical terms used in this World Mining article and in other solvent extraction literature.
Contactor
Device for dispersing and disengaging immiscible solutions; extractor. May be single stage, as in a mixer-settler, or multiple stage, as in columns and certain centrifuges.
Countercurrent extraction
Multistage extraction in which the aqueous and organic solutions flow in opposite directions.
Concentration or distribution coefficient
Extraction coefficient, E-A/O, stripping coefficient, S-O/A, representing the ratio of metal solute concentrations after contacting (equilibrating) an aqueous and organic solution under defined conditions.
Extraction or distribution isotherm
Graphical representation of isothermal equilibrium concentrations of a metal solute, in aqueous and organic solutions, over an ordered range of conditions in extraction (extraction isotherm) or stripping (stripping isotherm).
Extract
Organic phase after extraction (loaded solvent), or aqueous phase after stripping (loaded strip liquor); the solution into which transfer of a metal solute is effected; used as a verb to describe transfer of a metal solute between two immiscible liquids.
Extractant
Organic solution or the active organic solute.
Loaded organic
Organic solvent containing metal solute after contacting the aqueous feed liquor; the extract.
Loading capacity
Saturation limit of metal solute in organic or strip liquor.
Mixer-settler
Device for liquid-liquid extraction, comprising separate mixing and settling compartments.
Pulse column
Multistage contactor comprised of a column usually containing parallel, horizontal, perforated plates (disks) through which the aqueous and organic feed streams are advanced countercurrently a pulsing motion.
Raffinate
The liquid phase from which solute has been removed by single or multiple-stage contacting with an immiscible solvent.
Settling
Separation of dispersed immiscible liquids by coalescence and sedimentation.
Solvent
In liquid-liquid extraction, the liquid phase that preferentially dissolves the extractable solute from the feed. The term is often used to describe the organic phase.
Solvent extraction
Separation of one or more metallic solutes, from a mixture, by mass transfer between immiscible phases in which at least one phase is an organic liquid.
Stage
Single contact (dispersion and disengagement); sometimes refers to a theoretical stage which is a contact that attains equilibrium conditions.
Stripping
Removal of extracted metal solute from loaded organic extract; re-extraction; back extraction. Selective stripping refers to separate removal of specific metal solutes from an extract containing more than one metal solute.
 Glossary from “Application of Solvent Extraction” to Common Base Metals (a Review)
Glossary from “Application of Solvent Extraction” to Common Base Metals (a Review)
Department of Energy, Mines and Resources, Mines Branch, Ottawa, Quebec, Canada.
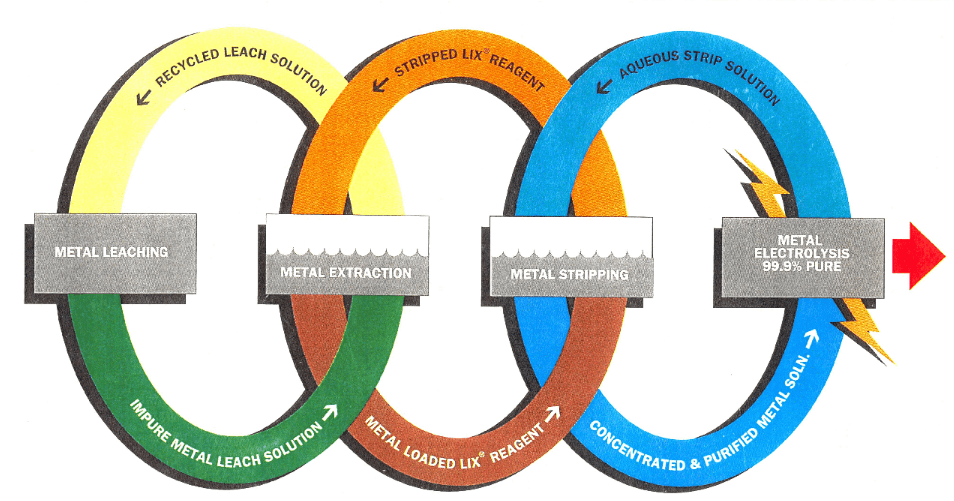
Solvent extraction of metals promises well to be the technique which will change the face of base metal mining and extractive metallurgy over the next decade.
Change mining because it makes possible the treatment of ores hitherto considered too low grade or too refractory for treatment by conventional methods.
Change extractive metallurgy because not only does it compare favorably in cost with leach precipitation and thermal smelting methods, but it also means that high purity saleable metal can be produced at the mine. The fact that it eliminates the smelter stage with its attendant atmospheric pollution problems will not be the least significant advantage of solvent extraction techniques in the coming years.
The application of solvent extraction to the recovery of copper has set the pace in this field. A number of articles in WORLD MINING have, drawn attention to this developing trend. (November 1968, p. 41, April 1969, pp 63, 79, May 1969, p. 37, December 1969, pp 34 to 37).
Now that the process has been proven technically and the economics have been shown to compare very favorably with those of cementation there is expected to be a large increase in the number of solvent extraction plants which are built. The low cost of the process has made the treatment of many low-grade oxide deposits economic and has also enabled copper to be recovered economically from tailing.
