The equipment which is used in solvent extraction plants for contacting the organic and aqueous phases and separating the same is varied. Most common to the industry is the mixer-settler unit which, in itself, can be of varied design; but many new innovations in contacting and separating equipment have been recently introduced or are now being studied.
Reagents
Each solvent extraction reagent must possess a number of properties or criteria before it can be economically used in a process to recover a given product. The value of the product will often dictate the degree to which a reagent must meet these criteria.
Listed below are some of the more important properties, according to Swanson and the possible impact each may have on the economics of a solvent extraction process:
- Capable of selectively extracting some ion or ions under specific conditions; excellent selectivity can result in the elimination of extraction or wash stages and can reduce expensive bleed streams,
- Kinetically fast in reaction; fast reactions result in smaller mixing equipment, thus a smaller reagent inventory and sometimes a lower mixing power equipment,
- Capable of reacting, reversibly; the reagent must be stripped or regenerated under conditions that allow the final product to be recovered economically,
- Physically and chemically stable to the conditions of use; an unstable reagent may degrade quickly and become ineffective, thus increasing reagent usage,
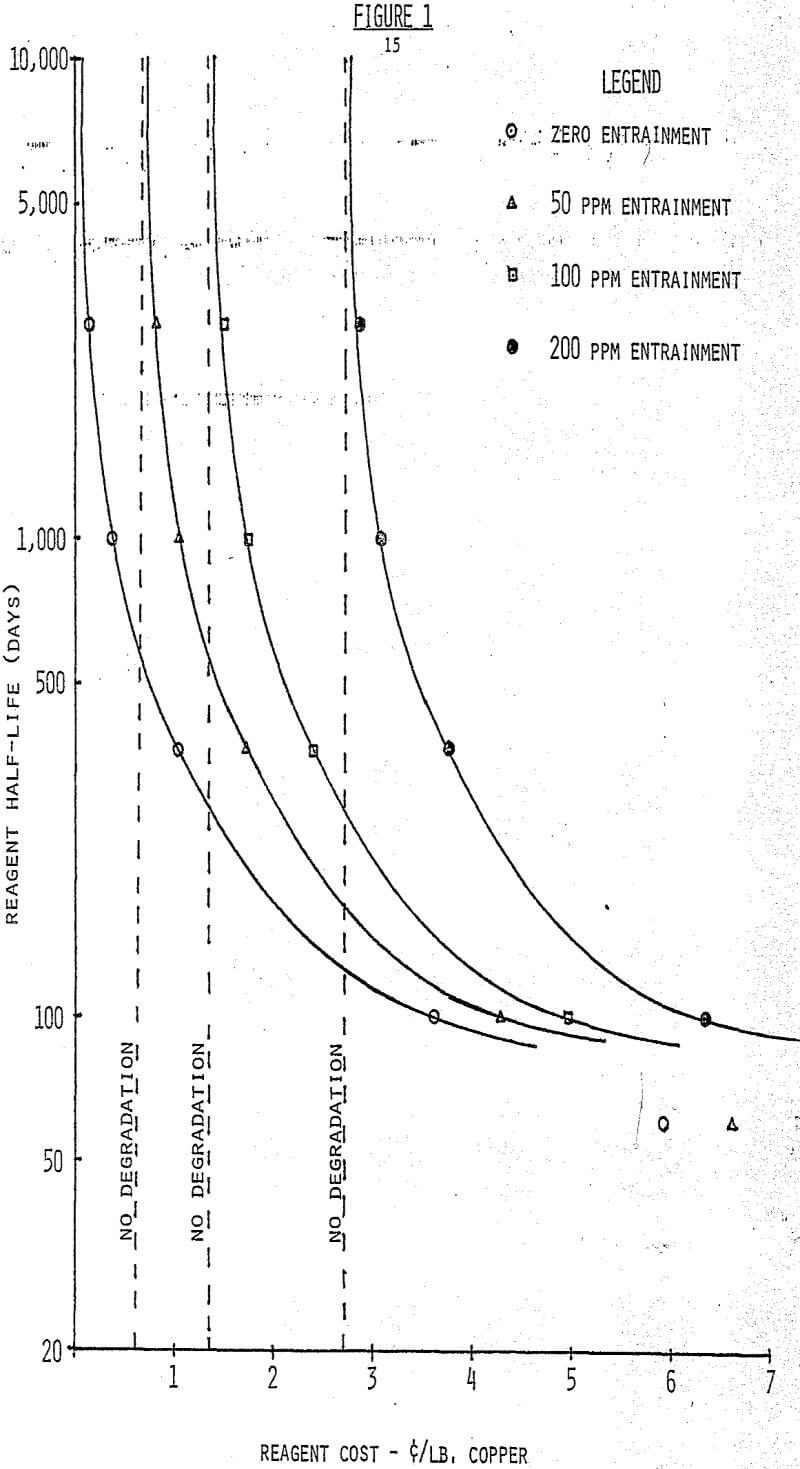
Entrainment should be noted as a general term relating to phase-separation and solubility, a source of reagent loss to an exiting aqueous stream. In the example, the following assumptions are made:
- Aqueous feed is 1000 GPM at 1.5 g/l Cu.
- Solvent extraction circuitry consists of three extraction and two strip stages.
- Total circuit organic inventory is 57,000 gallons.
- Reagent concentration is 10% in the organic phase
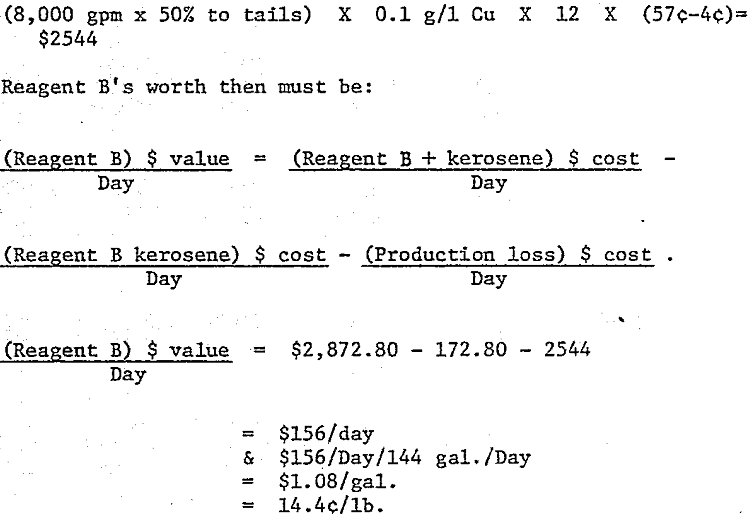
It follows from the above assumptions that the plant using Reagent A will produce 336,000 lbs. copper per day at an organic cost of $ 2,872.80 per day (0.855¢/lb), and Reagent B will yield 326,400 lbs copper per day at an organic cost of $2,872.80 per day (0.88(¢/lb). Reagent B not only costs a slight amount more in ¢/lb. to operate, but it also costs the operating company an additional $2,544 per day in lost cathode production. This is calculated as follows:
LIX 54 is another new reagent from General Mills Chemicals, Inc. It is described in an unpublished paper, and its properties make it a more economical reagent for extracting copper from some ammoniacal solutions. The economic advantages of LIX 54 in this system are several. It is capable of loading large quantities of copper such that the total flow in a given circuit is small. This equates to smaller mixers and settlers. LIX 54 does not load ammonia which in many cases must be neutralized or washed from the organic, thus reducing the operating cost of the circuit. Additionally,
Equipment
Some of these are listed as follows:
- Gravity Mixer-Settler
- Vertical contact column
- Centrifugal contactor-separator
- Graesser contactor
- Lurgi contactor-separator
- IMI mixer-settler
Although General Mills Inc. is not in the equipment business per se, we often work closely with various engineering groups and equipment manufacturers. Through these relationships, we have been able to observe and often work with various types of equipment in order to evaluate compatibility with a given reagent.
Systems
One of the most significant systems recently introduced to the industry is a result of some of the unique properties of LIX 34. This system is termed “parallel-series” and was discussed at the recent International Solvent Extraction Conference in Toronto.The parallel-series system is actually a unique flowsheet. Due to the highly selective nature of LIX 34 for copper over iron, a leach solution can be split into two or three equal parallel streams; and the organic phase can be advanced in series to a single extraction stage for each stream.
Another interesting system is described in a recent patent for zinc recovery by solvent extraction. This system tailors the aqueous media to the properties of the reagent for zinc solvent extraction. In effect, Di-2-ethylhexyl phosphoric acid, a well-known extractant, is a very selective and strong reagent for zinc in the sulfite system. It produces results from sulfite media that are markedly different than those from other acidic solutions..
The separation of nickel and cobalt in chloride media by solvent extraction has been described. Recent research has shown that iron can be separated as well by adapting a well-known technique to this system. The technique is that of pH adjustment within a given mixer, and the adaptation is similar except the EMF is monitored and adjusted rather than pH. In this fashion, iron can be extracted or stripped at many points within the solvent extraction circuit and the desired separations can be achieved.