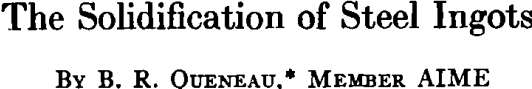Table of Contents
Steel has been chosen as the metal whose solidification will be used to tie in the principles discussed in the previous papers. Although steel is the most important practical example that could be chosen, its solidification is complicated by the presence of many elements added either intentionally or present as impurities. The liquid steel bath in an open-hearth furnace contains carbon, manganese, phosphorus, sulphur, and many residual alloying elements such as nickel, copper, and molybdenum. The bath also contains oxygen, the concentration of which is a function of the carbon content. Deoxidizers such as silicon and aluminum may be added either before or after tapping depending on the grade of steel being made. There are four types of steel ingots: Killed, semikilled, capped, and rimmed, and these differ from each other in their state of oxidation. Each type of steel has advantages in the production of specific steel products, and it should be emphasized that no one type should be considered superior to the others.
Fully deoxidized steels, known as killed
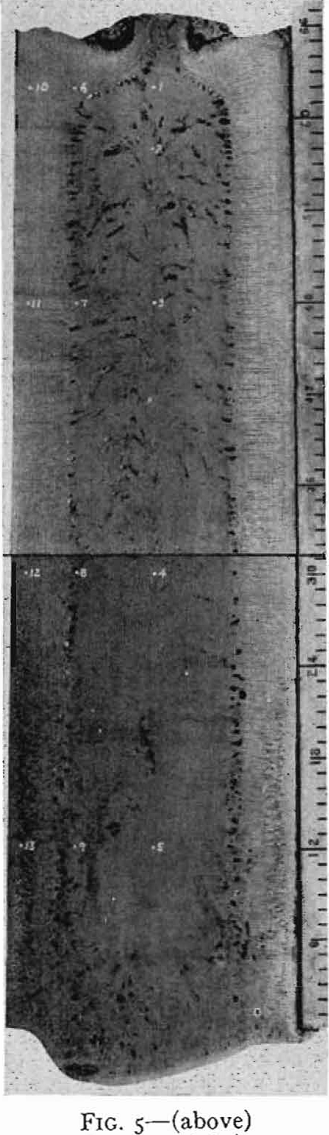
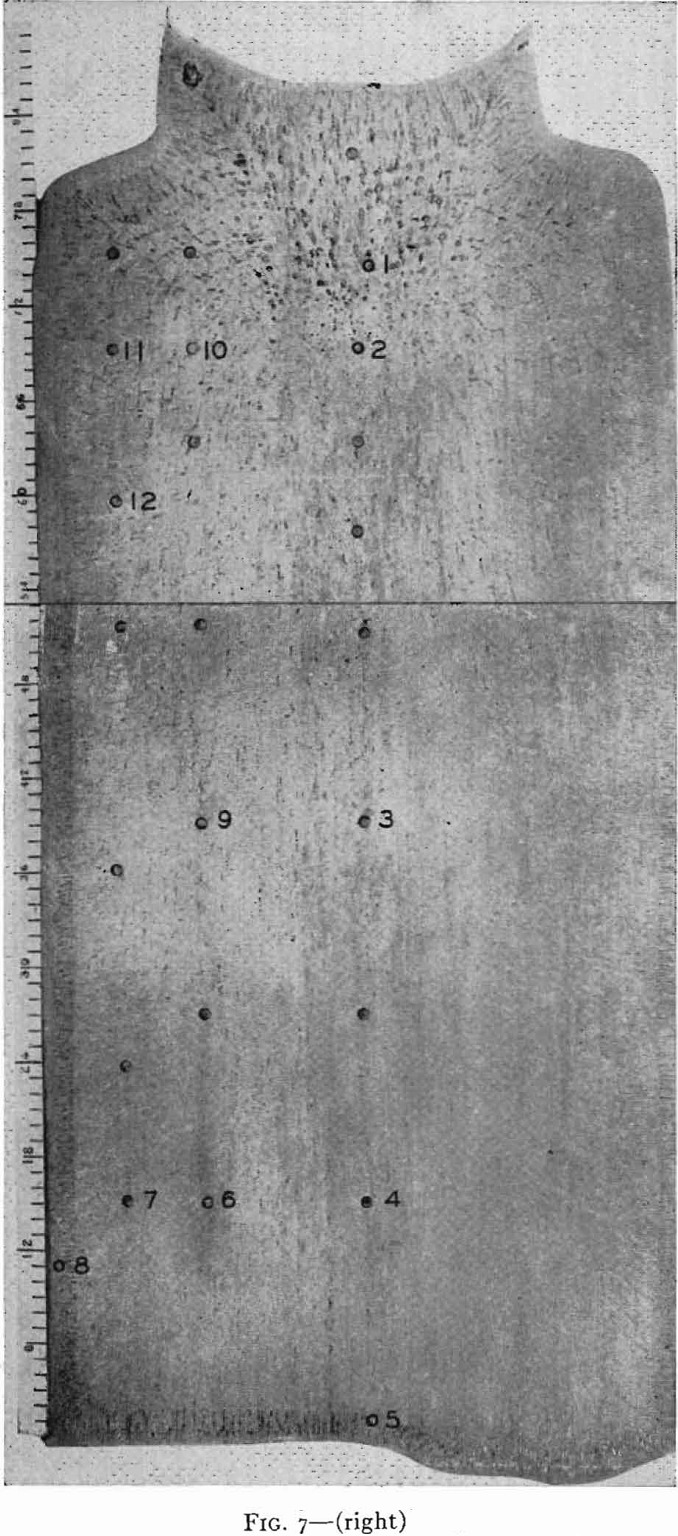
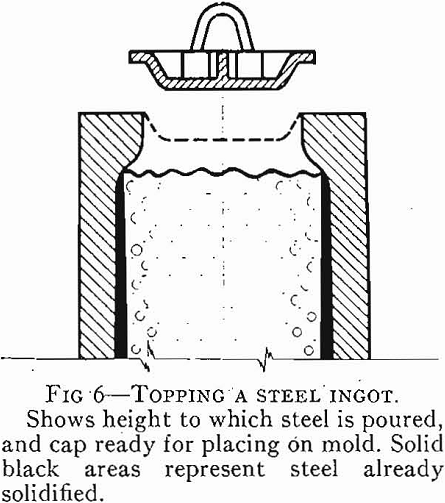
steels, have little or no gas evolution on solidification. When the steel solidifies in the mold, shrinkage occurs which causes a large void known as “pipe.”

Pipe, segregation, and inclusions are defects in the steel which have to be controlled by correct steelmaking practice and ingot mold design and cannot be remedied once the steel is solidified. Surface defects such as scabs, seams, and cracks can be removed from the rolled product by scarfing with oxy-acetylene torches, grinding with abrasive wheels, or chipping with air hammers, or by machine. This short outline of defects in steel has been given to emphasize that the bottleneck to the production of high quality steel can be said to be the problem of solidification of liquid steel. The metallurgical dream of pouring molten steel into
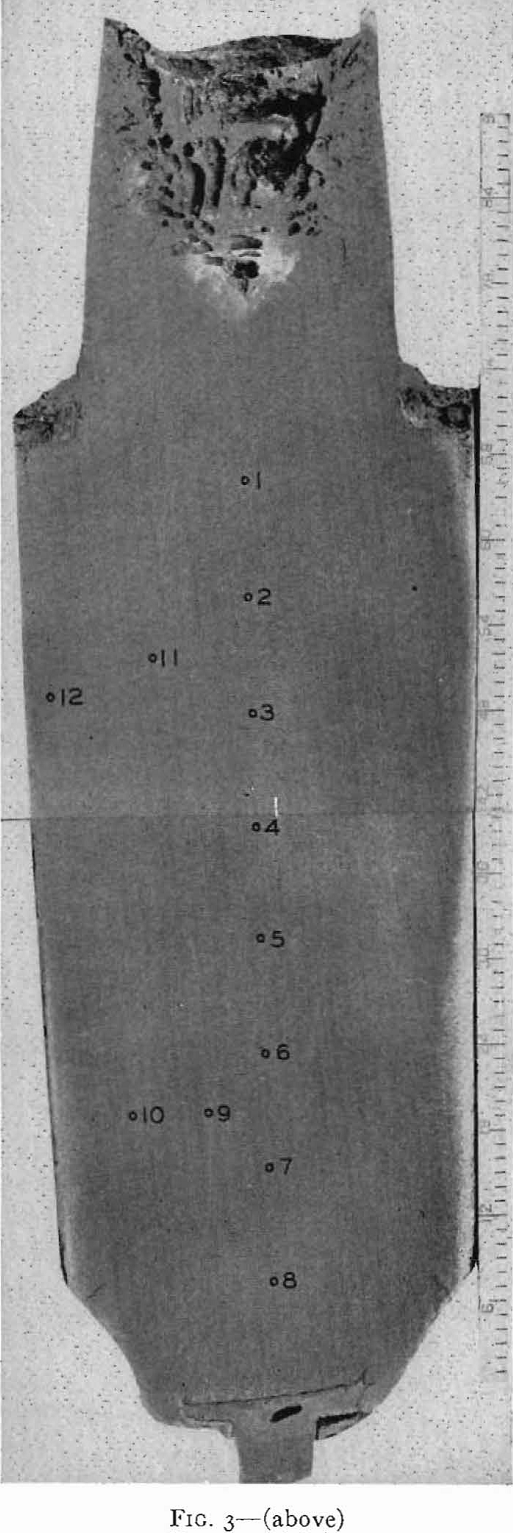
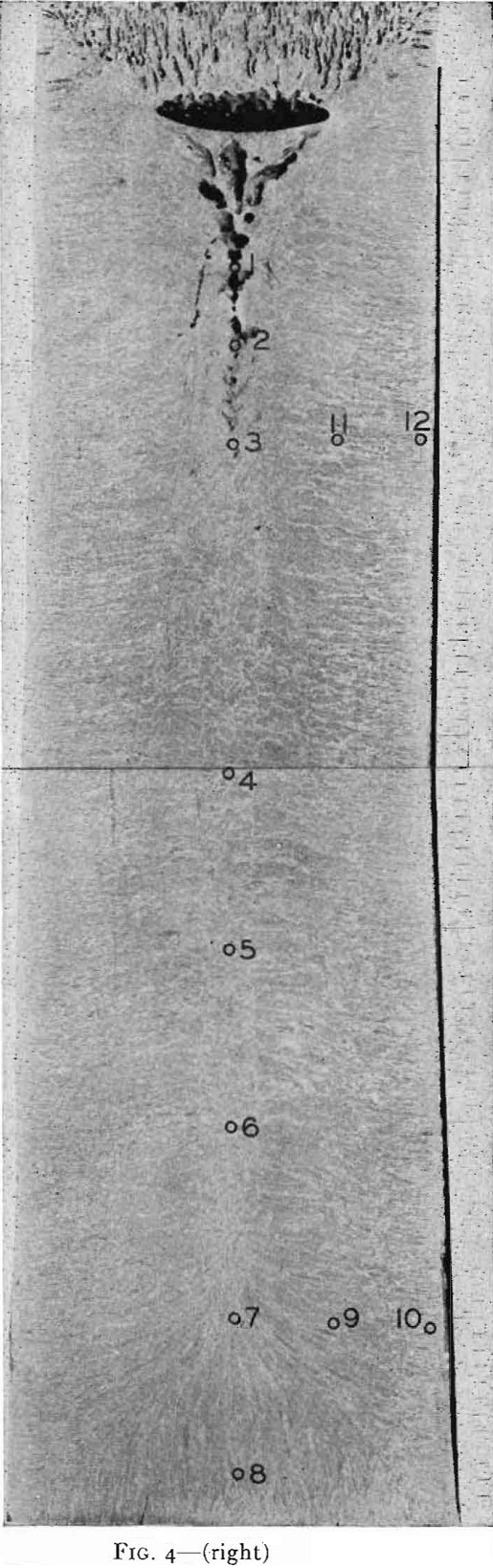
forming rolls or some other method of direct casting to semifinished product is a pleasant one not only from the viewpoint of quality, but also of cost. However, direct casting of steel presents many difficulties even in forming small bars, while casting slabs for rolling into sheet products appears to be insurmountable at the present time.
Rimmed Steels
Production and Structure of Rimmed Steels
In finishing a heat of rimmed steel in the open hearth, it is necessary to have the bath contain a large and controlled amount of oxygen. Since the analysis of the steel is the chief factor influencing the oxygen content, it follows that there is an optimum analysis for capped and rimmed steels which will vary somewhat from shop to shop but is normally about 0.08 pct C and 0.40 pct Mn. If the steel is ordered to the low side of this optimum, it is possible to add sufficient deoxidizer, usually aluminum, to the ladle to obtain the desired amount of rimming action in the molds.
are the direct opposites of semikilled and killed steels. In the latter steels, it is the lower carbon steels that give the most trouble from nonmetallic inclusions, the cleanliness of the steel increasing with increase in carbon content.
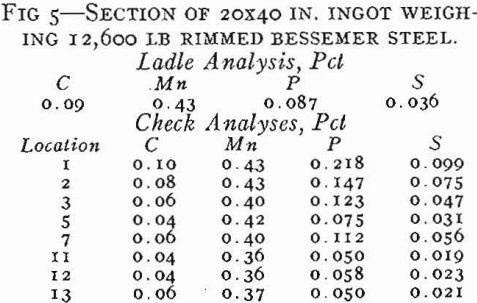
On tapping a rimming heat, coal and ferromanganese are added to the ladle as required to meet the ordered analysis. About ¼ lb Al per ton is added to the ladle on lower carbon grades, but the aluminum addition must be made sparingly so that the pourer can control the final deoxidation in the mold. On pouring rimmed steel into open top, big-end-down molds, a measured amount of aluminum shot is added uniformly throughout the filling of the mold. If the correct amount of aluminum is added, the characteristic rimming action will take place with the level of the ingot remaining constant or rising slightly. As the top surface gradually freezes in from the mold walls, the liquid center becomes smaller in size. It is customary to cover the top of rimmed ingots with a piece of heavy steel plate after a specified time interval to stop the rimming action. Such an ingot is shown schematically in Fig. 13b. There is a row of elongated primary blowholes near the surface in the lower half of the ingot, another row of deep seated spherical blowholes the full length of the ingot (frequently called secondary blowholes), and also large blowholes scattered throughout the core of the ingot.
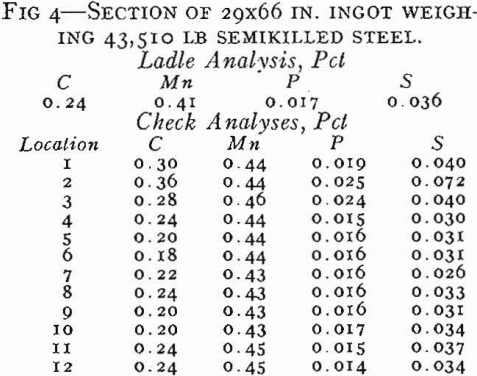
Segregation in Rimmed Steel
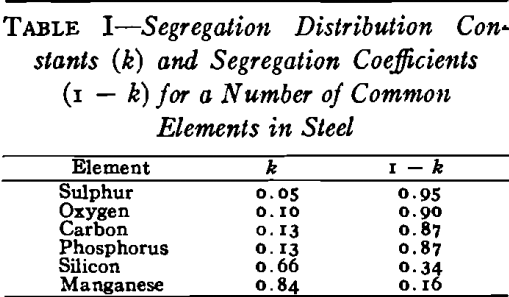
The basic cause of segregation is the lower solubility of impurities in solid than in liquid iron. The metal that first solidifies is lower in impurities than the liquid, and the tendency of the different elements to segregate in liquid iron can be expressed as a ratio between the concentration of the element in the first metal to freeze and the concentration of the element in the last metal to freeze.
Capped Steels
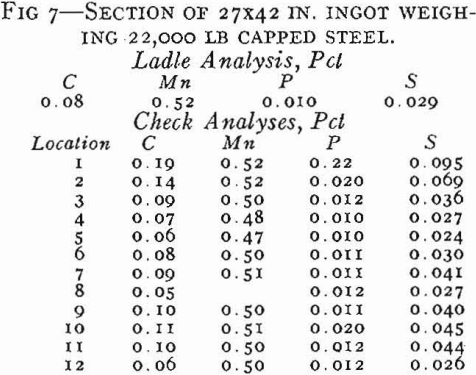
In capped steel, the deoxidation practice is such that the steel will rise in the molds. The rimming action in the molds is stopped as soon as the steel makes a seal against the cap, and the ingots therefore have a thin rim.
In general, the segregation in capped steels will be less than in rimmed steels because the short rimming time produces less concentration of impurities in the core. Another major advantage of capped steels as compared to rimmed is that taller ingots can be poured.
Semikilled Steels
As stated in the introduction, the aim in producing semikilled steels is partially to deoxidize the heat so that the central pipe cavity will be kept to a minimum by the formation of blowholes throughout the ingot body. A common practice therefore is to make no deoxidizing additions to the furnace and to add manganese required to meet the melting specifications in the ladle together with a variable amount of ferro- silicon or aluminum, or both, depending on the grade of steel.
Killed Steels
In the manufacture of killed steels sufficient quantities of deoxidizers are added to reduce the oxygen content below that necessary to form carbon monoxide. Hence, killed steels are usually considered to have no evolution of gas on solidification. This is not necessarily true, however, since both hydrogen and nitrogen are dissolved in steel in appreciable amounts and they may be evolved during solidification. The amount evolved will depend on the concentration at the liquid-solid interface and on the pressure, and it is possible for them to form blowholes in a manner similar to carbon monoxide.
Final deoxidation and adjustment of the analysis of killed steels is accomplished in the ladle with no additions being made to the molds. Ferrosilicon is added to the ladle to produce a final silicon content of about 0.15 to 0.30 pct. Aluminum usually is added in amounts varying from ¼ lb per ton to approximately 3 lb per ton. The steel normally is poured into big-end-up molds with refractory hot tops. The rate of pouring should be controlled carefully for each mold size. Slow pouring speeds tend to trap scum inclusions in the surface of the ingot, and surging of the steel frequently causes folds in the surface by liquid steel flowing down between the solidified skin and the mold wall. Fast pouring may cause excessive pressure within the ingot while the skin is thin and will produce skin ruptures and ingot cracks.
On solidification, the structure of a killed-steel ingot consists of a zone of small equiaxed crystals followed by a zone of columnar crystals nearly perpendicular to the mold wall. In the remainder of the ingot, the crystals again are randomly oriented. These three zones are common to all killed steels, though their relative extent and position vary with composition of the steel and pouring conditions. In the chill zone, there is little time for segregation and the steel is nearly uniform in composition.