Slags generated by the ferrous-metals industry can be used to produce high-tech ceramic materials. The properties of these ceramics can be controlled by judicious choices of composition, additives, and processing parameters. Standard methods of materials handling, such as comminution and mixing, are as applicable to slag as to any other mineralogic raw material.
Materials and methods
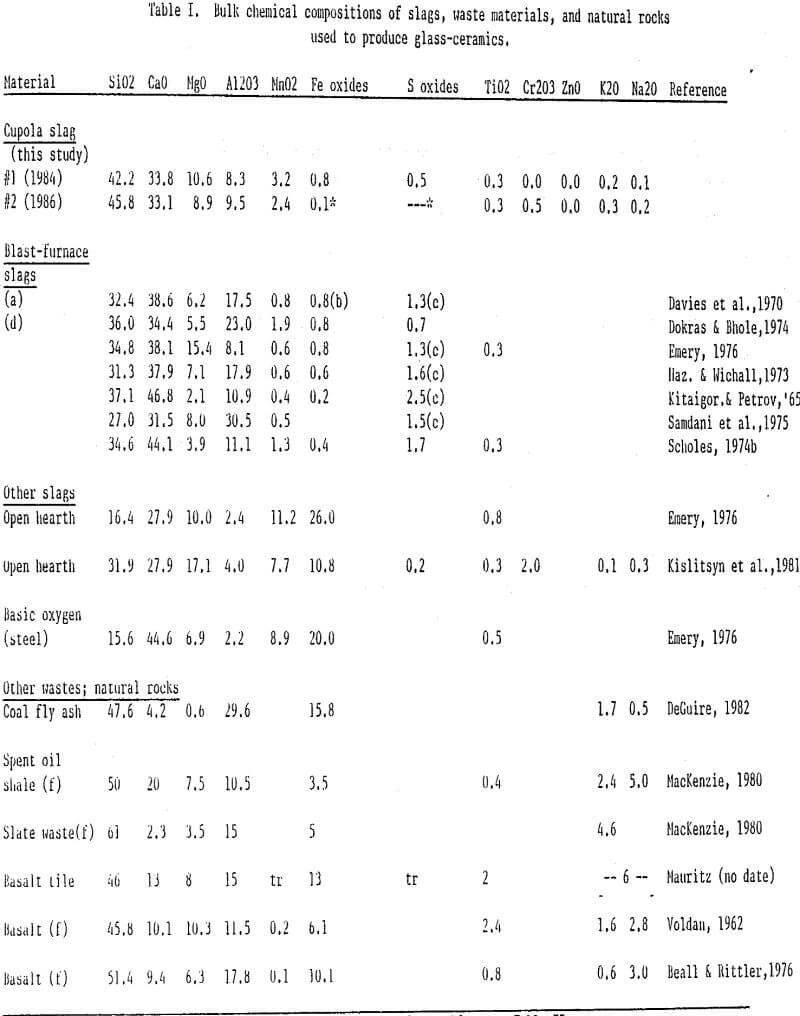
The cupola slags investigated in this study were obtained from a foundry that produces cast iron pipe and fittings. Samples were collected and combined daily over a period of weeks in 1984 and again in 1986. During this period, production methods were nominally unchanged, and average compositional differences reflect differences which could be expected over time if the foundry were used consistently as a raw-materials source. The slag chunks were crushed to approximately 1-5 mm using jaw and plate crushers. This granular material was then ball-milled until the largest particles were approximately 0.5 mm.
Results and discussion
The slag glasses are nearly opaque except at thin edges, where they may appear dark green, dark brown, or streaky, with mixed colors and occasional clear streaks. After heat treatment the ceramics lose their vitreous luster and appear almost waxy on fresh surfaces, although they still exhibit conchoidal fracture. The color of the heat-treated ceramics ranges from nearly black to very dark brownish-gray, and they may show evidence of slight sagging or surface softening.
Within glasses made from both slags there appear streaks of transparent, clear to pale brown or green material, which does not lend itself to bulk crystallization; i.e., very few crystals originate within these streaks. However, after heat treatment these streaks are often filled in with large feathery dendritic growths originating either at the boundaries or at the few nuclei which do appear, suggesting that the nucleation-promoting species is impoverished in this region.
The origin of these streaks was at first thought to be related to atmospheric exposure and possible oxidation during pouring, because areas exposed and later incorporated during the pour behaved similarly to areas forming the final surface. However, melts from slag 2, which were not stirred, appear to have more and larger streaks. One melt, cooled and annealed in the crucible, showed a thick (0.5 cm) clear layer at the top, suggesting either a major effect of oxidation, or some sort of compositional separation. This thick clear layer, drawn out of the crucible on the upper surface of the molten glass, probably accounts for the presence and distribution of the clear streaks within the sample bars.
Mineral compositions in glass-ceramics derived from slag 1 were identified by x-ray diffraction (XRD). Two-hour single-step heat treatments produced bustainites (pyroxenoids) at treatment temperatures of 880 C – 920 C, diopside (clinopyroxene) above 990 C, and mixtures of these at intermediate temperatures. The identification of bustamite [(MnCa)3Si3O9]„ is still tentative, as the XRD patterns are not a perfect match, and the manganese level in the bulk slag is quite low. However, it is possible that the phase may be a mixture.of bustamite, ferrobustamite [Ca(Mn,Ca,Fe)3Si3O9] and bustamite-like solid solutions incorporating other cations.
Projected uses of slag glass-ceramics
The Soviets have been using slag glass-ceramics in the construction industry for some time. Large slabs, formed continuously by a float glass process and heat treated in a single step as the slab passes through a gradient furnace, can be mounted in the same manner as plate glass. Smaller items such as stair treads and floor tiles can be cast with safety-grip surfaces and installed as normal building materials. The glass-ceramic’s heat resistance and chemical inertness can be used to advantage in processing areas where chemicals and hot materials are handled.
Foamed glass-ceramics can be made by dissolving gas in the melt under pressure (e.g., adding ingredients which decompose to produce a gas at melt temperatures, and melting in closed crucibles); when poured, the gas expands into a pumice-like foam. Subsequent heat treatment promotes crystallization of the glassy bubble walls. The resultant glass-caramics are very light; this plus their resistance to rot makes them ideal for construction on and around water.