Table of Contents
The slag from the blast furnaces having been granulated, a representative sample is taken to the sample mill. It is then dried, crushed through a 100-mesh screen, intimately mixed, and packeted for assay purposes.
METHOD OF ANALYSIS
INSOLUBLE
Weigh 1 grm. of the finely-pulverized slag into a 3½-in. casserole, moisten with a little water to prevent sticking, cover with a clock glass, and add 20 cc. of hot acid (1 HNO3, 3 HCl, 4 H2O). Evaporate slowly to dryness on a hot plate and bake (without overheating) until all the gelatinous silica is dehydrated. Cool, add 20 cc. HCl (1-1), and boil gently for several minutes; filter into a 300-cc. beaker, cleaning out the casserole thoroughly with a rubber-tipped glass rod. Wash residue with hot distilled water and transfer it to a No. 1 porcelain crucible. Dry, ignite, cool in a desiccator and weigh as total insoluble matter.
Dilute filtrate to 200 cc. and neutralize some of the excess HCl with NH4OH. Warm, and pass H2S for 15 min. Filter and wash with hot water. Boil off H2S, oxidize with 5 cc. HNO3, reduce bulk of the solution by evaporation to about 100 cc., and transfer to a 250 cc. flask.
SILICA
Fuse insoluble residue over a blast lamp in a platinum crucible with three or four times its weight of anhydrous carbonate of soda, for 10 to 15 minutes, or until decomposition is complete. Cool, and extract fusion in an 8-in. evaporating dish with HCl (1-1); rinse crucible and cover with distilled water, and evaporate to dryness over a water bath. Take up dry mass with 10 cc. strong HCl, and then 50 cc. of hot water; heat to boiling, and filter into the flask containing the original filtrate. Detach adhering silica with a rubber- tipped glass rod. Wash well with hot water. Make the solution up to the 250-cc. mark, cool, and mix thoroughly. Transfer filter paper with silica to a No. 1 porcelain crucible, dry, ignite, cool in a desiccator, and weigh as SiO2.
carbonate of soda, for 10 to 15 minutes, or until decomposition is complete. Cool, and extract fusion in an 8-in. evaporating dish with HCl (1-1); rinse crucible and cover with distilled water, and evaporate to dryness over a water bath. Take up dry mass with 10 cc. strong HCl, and then 50 cc. of hot water; heat to boiling, and filter into the flask containing the original filtrate. Detach adhering silica with a rubber- tipped glass rod. Wash well with hot water. Make the solution up to the 250-cc. mark, cool, and mix thoroughly. Transfer filter paper with silica to a No. 1 porcelain crucible, dry, ignite, cool in a desiccator, and weigh as SiO2.
FEO – CAO – MGO
Pipette 100 cc. of solution from the flask into a 300-cc. cylindrical beaker, add 1 grm. of NH4Cl, 10 cc. of bromine water, and make alkaline with NH4OH. Boil for a few minutes, and add another 10 cc. of bromine water and again boil. Filter into a 600-cc. beaker and wash well with hot water. (Reserve filtrate for CaO and MgO.) Dissolve the precipitate out of the filter with hot HCl (1-1), catching the solution in the original beaker, and at the same time dissolving any ferric hydroxide adhering to the sides of the beaker. Heat to boiling, and then reduce with SnCl2 solution, add 20 cc. of saturated HgCl2 solution, and titrate with standard K2Cr2O7 solution. (1 cc. = 0.01 grm. FeO.)
cc. used x 5/2 x 0.01 x 100 = % FeO.
Heat filtrate reserved for CaO and MgO to boiling, add 10 cc. saturated ammonium, oxalate solution, and continue boiling for five minutes longer. Allow to stand in a warm place for 15 minutes. Filter and wash the precipitate until the washings give no test for oxalate. (Reserve filtrate for MgO.) To the original beaker add 20 cc. H2SO4 (1-1) and 200 cc. boiling water, and to this add the filter paper containing the calcium oxalate precipitate, stir well, then titrate immediately with standard KMnO4 solution. (1 cc. = 0.005 grm. CaO.)
cc. used x 5/2 x 0.005 x 100 = % CaO.
Pass H2S into the filtrate reserved for magnesia for 15 minutes, boil off H2S, and at the same time reduce the bulk to about 75 cc. Filter through a double filter paper and wash with hot water several times. Make the filtrate just acid with HCl, bring to boil, and add a few cc. of a solution of microcosmic salt while boiling, cool, add one-third the bulk of a 10% solution of NH4OH. Allow to stand 12 hours, stirring at intervals. Filter through a double filter paper and wash with 2½% ammonia water. Transfer precipitate to a No. 1 porcelain crucible. Dry and ignite, first at a low temperature and finally at a bright red heat, to constant weight. Weigh as Mg2P2O7.
Weight of precipitate x 5/2 x 0.3624 x 100 = %MgO.
MNO
Pipette 50 cc. of solution from the flask into a 500-cc. cylindrical beaker, add 5 cc. HNO3, and bring to boil. Remove from heat and add an emulsion of zinc oxide and water until iron is precipitated. Make bulk up to 300 cc. with boiling water, and titrate with standard KMnO4 solution. (1 cc. = 0.0038 grm. MnO.) Titration times 5 x 0.0038 x 100 = % MnO.
AL2O3
Wash out the remaining 100 cc. of original solution from the flask into a 600-cc. beaker. Add 15 cc. of a saturated solution of microcosmic salt, and then add ammonia cautiously and with constant stirring until a slight permanent precipitate forms. Now add 5 drops concentrated HCl, which should dissolve the precipitate and leave a clear solution, then add with constant stirring 20 cc. of a saturated solution of Na2S2O3. Dilute to 300 cc., cover, and heat to boiling. When boiling add 20 cc. of a solution consisting of 100 grm. sodium acetate, 200 cc. acetic acid sp. gr. 1.04, and water to make 500 cc. Boil the solution 10 minutes longer. Let the precipitate settle, filter, and wash well with hot water. Now transfer the precipitate to the original beaker, dissolve in HCl, filter, and re-precipitate as described above. Dry and ignite precipitate, cool in a desiccator, and weigh as AlPO4.
Weight of precipitate x 5/2 x 0.4185 x 100 = %Al2O3.
ZINC
Weigh 1 grm. of slag into a 3½-in. casserole, cover with a clock glass, add 15 cc. strong HNO3, and heat over a Bunsen flame until red fumes of nitrogen peroxide have all been given off. Add 1 grm, of finely-powdered KClO3, bring to boil over a Bunsen flame, using a rotary motion, and then evaporate slowly on asbestos hot plate almost to dryness. Add 30 cc. strong HNO3 and a little KClO3 heat to boiling, and allow to stand on hot plate 10 minutes. Filter through asbestos pulp and glass wool, detaching adhering insoluble material with a glass rod. Wash twice with HNO3 (1-1) and several times with hot water. Add 5 grm. of cream of tartar and make alkaline with ammonia. Heat to 70° C. and titrate with standard K4Fe (CN)6 solution, using glacial acetic acid as an outside indicator.
K4Fe(CN)6 solution (1 cc. = 0.01 grm. Zn.)
Alternate Method.—Weigh 1 grm. of slag into a 10-oz. conical beaker, add 5 cc. HCl, and evaporate gently to dryness; continue heating at a low temperature until silica is dehydrated. Cool, add 10 cc. concentrated HNO3, warm gently, then add 1 grm. powdered KClO3, and evaporate to dryness. Allow to cool, then add 50 cc. hot water and 7 grm. NH4Cl. Stir with a rubber-tipped glass rod to ensure disintegration of the mass, then add 15 cc. NH4OH and ½ grm. Na2O2, cover, and boil for 2 minutes. Filter into a 600-cc. beaker and wash several times with hot 1 % NH4Cl solution. Re-dissolve the precipitate with as little HCl as possible into the original beaker, and wash residue in filter paper with hot water until free from ferric chloride. Make solution alkaline with NH4OH, cool, and add 3 or 4 grm. of Na2O2. Boil for 2 minutes and filter into the beaker containing the first filtrate. Wash with hot 1 % solution of NH4Cl. Make solution just acid with HCl, add 5 grm. granulated lead, and boil for a few minutes until copper is precipitated. Make alkaline with NH4OH, add 30 cc. of tartrate emulsion, heat to 70° C., and titrate with standard K4Fe(CN)6 solution (1 cc. = 0.01 grm. Zn), using glacial acetic acid as an indicator.
LEAD
Weigh 5 grm. of slag into a 4-in. casserole, moisten with water, and decompose with 30-40 cc. of hot acid (1 HNO3, 3 HCl, 4 H2O). Evaporate slowly to dryness and bake to dehydrate gelatinous silica. Cool, add 20 cc. strong HCl, and boil for 2 or 3 minutes ; dilute with hot water and filter into a 600-cc. beaker, keeping as much as possible of the residue in the casserole. Re-treat residue several times with strong HCl, each time diluting and filtering into original filtrate. Finally, transfer residue on to the filter paper and thoroughly wash with hot water. The filtrate, which is about 300 cc., is nearly neutralized with NH4OH, leaving just sufficient acid to keep the iron in solution. Warm and pass H2S for at least 30 minutes. Filter and wash with H2S water. Dissolve precipitate in dilute HNO3 (1-1), add 10 cc. strong H2SO4, and evaporate to fumes. Cool, dilute with cold water, allow to settle, and filter off PbSO4. Wash twice with cold water, then place filter paper and PbSO4 in the original beaker, add 20 cc. ammonium acetate solution, 150 cc. hot water, boil, and titrate with standard ammonium molybdate solution (1 cc. = 0.01 grm. Pb). As the above method is too long for ordinary routine work, a much quicker method is substituted.
Alternate Method of Assaying
Weigh 1 grm. of slag into a 3½-in. casserole, moisten with water, and add 20 cc. hot acid (1 HNO3, 3 HCl, 4 H2O). Evaporate to dryness without a clock glass and bake carefully. Add 20 cc. and evaporate until only a few cc. are left. Cool, add a little cold water, and dilute with boiling water. Allow to stand until sulphate of iron is dissolved and then filter. Wash twice with hot water. Place precipitate and filter paper in a beaker containing ammonium acetate and hot water, boil, stir well, and titrate with standard ammonium molybdate solution.
SULPHUR
Weigh 1 grm. of slag into a 3½-in. casserole, cover with a clock glass, and add 20 cc. of a solution of HNO3 saturated with KClO3. Evaporate slowly to dryness on asbestos hot plate, cool, add 3 grm. anhydrous Na2CO3, 20 cc. hot water, and boil for 5 minutes. Filter into a 600-cc. beaker containing 15 cc. HCl. Detach adhering residue from the casserole with a rubber-tipped glass rod. Wash precipitate in filter paper four or five times with boiling water. Heat the filtrate to boiling, add 5 cc. of a saturated solution of BaCl2, and continue boiling for 5 minutes longer. Allow precipitate to settle, filter, and wash with hot water until free from BaCl2. Place precipitate in a No. 1 porcelain crucible, dry, ignite, cool in a desiccator, and weigh as BaSO4. Factor for S = 0.1373.
CHLORINE
Weigh 5 grm. of slag into a 300-cc. conical beaker, add 5 grm. Na2CO3 and 100 cc. hot water. Boil for 10 to 15 minutes. Filter into a 500-cc. beaker containing 20 cc. HNO3. Wash residue five or six times with hot water. Heat the filtrate to boiling, add a few cc. of AgNO3 solution, and continue heating until the precipitate of AgCl has coagulated. Filter and wash until free from AgNO3. Place filter paper containing precipitate in a small scorifier, dry, cover with a little granulated assay lead and litharge, and scorify. The resulting lead button is then cupelled and the silver prill weighed. Factor for chlorine = 0.3287.
COPPER – ARSENIC – ANTIMONY
Weigh 10 grm. of slag into a 4-in. casserole, cover with a clock glass, add 50 cc. HNO3, and heat until thoroughly decomposed. Add 40 cc. H2SO4, and evaporate to strong fumes. Cool, dilute, add a few grams of tartaric acid, and boil until all soluble sulphates are dissolved. Allow to settle, filter into an 800-cc. cylindrical beaker, retaining residue in the casserole. Wash by decantation until free from tartaric acid. The residue is now decomposed with 30 to 40 cc. HCl, fumed again with 20 to 30 cc. H2SO4, diluted, tartaric acid added, boiled, and filtered as previously. To the filtrate add NH4OH, until most of the excess acid has been neutralized. Warm on hot plate and pass H2S for 30 minutes. Allow to stand in a warm place for several hours, or preferably overnight. Warm and pass H2S again for 20 minutes, allow to settle, filter, and wash well with H2S water. Open the filter paper, and by means of a jet of hot water wash the precipitate into a 300-cc. beaker; dissolve any precipitate adhering to the filter paper with a little hot dilute HNO3. Make the solution alkaline with NaOH, and warm to dissolve the sulphides of arsenic and antimony. Dilute with hot water to 100 cc., add 5 cc. Na2S solution, and allow to stand in a warm place for 15 minutes. Filter and wash with warm dilute Na2S solution. (Reserve filtrate for arsenic and antimony.) Dissolve the precipitate in dilute HNO3, filter off sulphur, make alkaline with NH4OH, cool, and titrate with standard KCN solution (1 cc. = 0.005 grm. Cu).
Make the filtrate reserved for arsenic and antimony slightly acid with HCl, pass H2S for 30 minutes, allow to stand in a warm place for 30 minutes longer. Filter and wash with H2S water. Dissolve the precipitate off the filter paper with a little hot dilute NaOH solution, add 1 grm. of powdered KClO3, and make distinctly acid with HCl. Allow solution to digest on hot asbestos plate without boiling until the sulphides of arsenic and antimony are decomposed and the solution is free from chlorine. Filter off sulphur, add 1 grm. tartaric acid. Make just alkaline with NH4OH, add 10 cc. “ magnesia mixture,” and one-third bulk of 10% NH4OH. Allow to stand 12 hours, stirring well at frequent intervals. Filter on to a weighed Gooch crucible, using the filter pump. Wash with 2½% NH4OH until free from chlorides. Dry at 100° C., and ignite in a muffle, first at a dull red and then at a bright red heat. Cool in a desiccator and weigh as Mg2As2O7.
Factor for arsenic = 0.4827.
Boil off excess of ammonia from the filtrate from the arsenic, and reduce the bulk to about 100 cc., make acid with HCl, warm, and pass H2S for 20 minutes. Filter on to a weighed Gooch crucible, wash well with hot water, heat in a current of CO2 at 300° C. for 1 hour. Cool to 100° C. in CO2, and finally in a desiccator. Weigh as Sb2S3.
Factor for antimony = 0.7146.
SILVER
The following charge is used :—

Sufficient sulphur is generally present to reduce the lead button; if not, use a little flour. The fusion takes 25 minutes, and a fairly high temperature is required. A small cupel is used for cupellation of the resulting lead button.
GOLD
The following charge is used :—

A fairly high temperature is required for the fusion. The above charge gives a button which may be cupelled directly in a large cupel. The resulting silver prill is parted with HNO3 in the usual way.
Notes on the Method.—Blank determinations should be carried out along with the assays. Should the slag be very low in lead, copper, arsenic, and antimony, separation of the Group 2 metals may be dispensed with without affecting the results.
FEO – CAO – MGO
Bromine water is added to the ammoniacal solution for precipitation of the manganese. As it is essential for all the manganese to be separated at this stage, two lots of bromine are added to ensure this, result. Should the manganese not be completely precipitated it will contaminate the Mg2P2O7. at a later stage. It is not essential to separate the zinc before precipitating the magnesia, but it is advisable, as some zinc may be precipitated along with it if this is not done.
ZINC
The end point obtained in the first method for zinc is a greenish colour, due to the large amount of iron in the slag.
In standardizing the solution of K4Fe(CN)6 for this method, iron wire approximately equivalent to the iron content of the slag is added.
Alternate Assay Method
In some cases the slag under treatment is not entirely decomposed by the ordinary acids, and the residue requires further treatment with HF and dilute H2SO4 for complete liberation of the zinc.
TARTRATE EMULSION
450 grm. cream of tartar, 45 cc. of a 10 % solution of neutral ferric chloride, made up to 3 litres with distilled water.
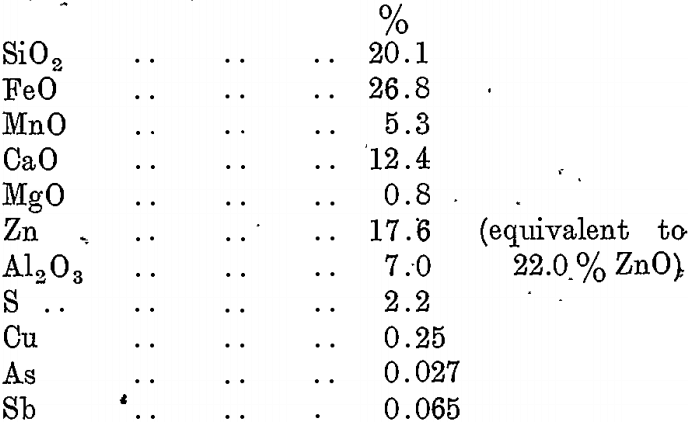
A typical analysis of lead blast-furnace slag as produced at the B.H.A.S. Pty. Ltd., Port Pirie, is as follows :—
Cleaning Slag
The slags of very rich ores may retain enough gold to necessitate further treatment. The slag is roughly crushed and fused with 1 A.T of litharge, 15 to 20 grains of charcoal and a little carbonate of soda, the same crucible being used again, or the slag may be fused and a mixture of litharge and charcoal thrown on to its surface. If any regulus or speiss forms during the first fusion it must be preserved with special care and refused, charcoal being reduced or omitted. The button of lead from the second fusion is cupelled with the first button, or alone; the slag from the second fusion is almost always poor enough to be thrown away.
H. L. Sulman considers that it is not necessary to pour and regrind the slag before “ washing ” it. Instead of this he uses a method similar to that adopted in “cleaning” scorification slag. When the crucible contents have fused and become quite tranquil, he stirs in first a little more oxide of lead and then a pinch of carbon, and thus makes a quick “ washing” form part of the ordinary operation.
