The ores from which silver and gold are recoverable by flotation divide themselves naturally into two general processing classes:
- ores in which the valuable minerals are those of the base metals, the precious metals being incidental constituents, and
- ores in which the gold and silver are of primary importance, the base-metal minerals, if present, being of little or no value.
The first class includes copper, lead, lead-zinc, copper-zinc, and copper-lead-zinc ores. Both gold and silver may be present, the gold values being usually associated with chalcopyrite and pyrite, and the silver with galena and tetrahedrite. Flotation follows ordinary standard methods, modified as may be necessary to bring up the precious metals in the concentrates where they will be of most value. Thus, in a lead-zinc ore, silver is more valuable in the lead than in the zinc concentrate. Similarly, in a copper or lead ore carrying gold associated with pyrite, it might be profitable to make an iron concentrate containing the gold. If the gold and silver can only be brought up in the desired concentrate by contaminating it with unwanted mineral, the mode of procedure must be determined by the economics of the case. Supposing, for example, that the recovery of the gold involved lowering the grade of a copper concentrate with unwanted pyrite, the fall in the value of the concentrate due to the presence of the pyrite must be more than counterbalanced by its rise in value due to the precious metal content if the operation is to be profitable.
Of the second class of ores carrying gold and silver values, the greater proportion consists of those in which the precious metals are associated with pyrite and other iron sulphides, arsenopyrite, or stibnite; “free-milling” ores, which contain a negligible amount of associated sulphide minerals, are not quite so common. The treatment of the first type of ore depends on the manner in which the gold and silver are associated with the sulphide minerals. The cyanide-agitation process is usually the most suitable method of extraction, but it often necessitates grinding to a fine size to release the gold and silver. Should it be possible to obtain a good recovery by flotation in a concentrate carrying most of the pyrite or other sulphide minerals, it may prove more economical to adopt this method, regrinding only the comparatively small bulk of concentrate previous to the cyaniding operation. Flotation may also prove to be the more economical process for an ore containing such minerals as stibnite, copper-bearing sulphides, tellurides, etc., which require roasting before cyanidation, since it reduces the tonnage passing through the furnace to a fraction of the original feed with considerable reduction of working costs in consequence. Even when the recovery of the gold and silver values from such ores by flotation is low, it may still be advantageous to float off the minerals which interfere with cyanidation, roasting and cyaniding, or possibly smelting, the concentrate for the extraction of its precious metal content; this is followed by cyanidation of the flotation tailing, which operation will then be simpler and cheaper because of the previous removal of the cyanicides.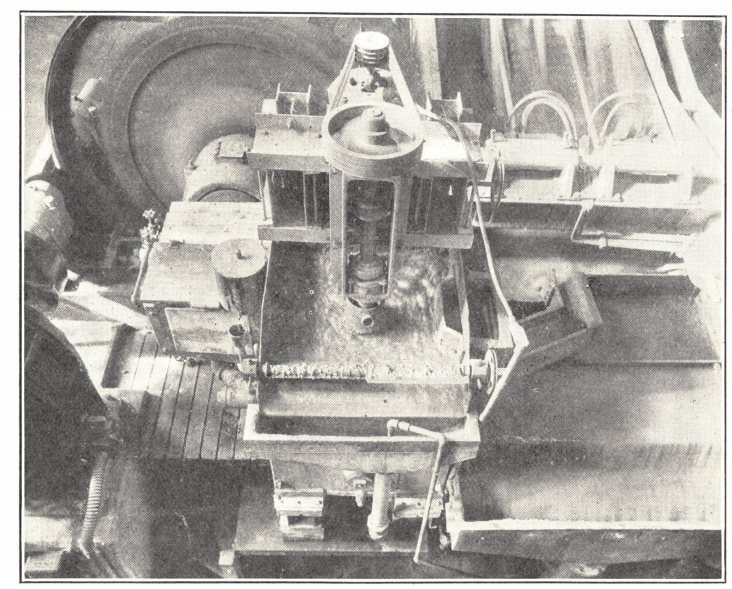
The treatment of “ free-milling ” ores—i.e., those in which most of the valuable mineral is free gold, usually consists of amalgamation or concentration on blanket or corduroy “ strakes ”, followed by cyanidation. Since treatment with cyanide solution involves either classification of the sand from the slime with a separate cycle for each product, or else “ all- sliming ” of the whole tonnage, it seems likely that in the future these methods will be replaced by flotation. A flotation plant will take unclassified pulp at a size which can be as coarse as is consistent with the liberation of the bulk of the minerals from the gangue, producing a concentrate for cyanidation that is seldom more than 10% of the tonnage milled, and often less. Moreover, it has the following advantages : The process is simpler, the capital cost of the installation is less, and the operating costs are likely to be lower.
Free gold and gold-bearing iron sulphides, though they tend to float slowly, can be brought up readily enough under the correct conditions with modern xanthate and aerofloat reagents. It is best, however, to recover as much of the precious metals as is possible in the grinding circuit by amalgamation or concentration on strakes in order to prevent its accumulation in the classifier; otherwise an occasional rush of material from the grinding section, due to some fluctuation there, may carry gold that is too coarse to float into the flotation circuit, with the result that such values pass out in the tailing and are lost. In this connexion, the use of a flotation cell in the grinding circuit, as described earlier in the chapter, has been employed with great success at the plant. The general arrangement of one of the five units is shown. The discharge from the tube mill is first passed through a 4-mesh screen to separate out coarse material, which is laundered direct to the classifier. The remainder of the pulp, diluted to give a W/S ratio of 1/1, passes to a Denver “ Sub-A” Flotation Cell, from which 60% of the gold and silver in the feed is removed as a concentrate. The tailing flows without loss of head to the classifier. Reagents are added at the tube mill feed box.
The bottom of each cell is fitted with a small hydraulic cone, situated immediately under the impeller. Here most of the gold that is too coarse to float collects instead of accumulating elsewhere in the tube mill circuit, the swirling action of the impeller apparently exerting a classifying effect which prevents the settlement of all but the heaviest particles. Once every 24 hours the tube mill discharge is sent direct to the classifier, and water is passed instead through the isolated flotation cell in order to clean out as much of the gangue as possible. The contents of the cone are then removed through a plug valve. Some 400 lb. of rich material is obtained in this way every day from the five cells, bringing the recovery of gold and silver in the grinding circuit up to 75%.
This installation was one of the first large plants to prove the value of flotation in the treatment of gold ores. At the outset, when the ore to be milled was mined at or near the surface, 80% of the values were recoverable by amalgamation, and a 90% recovery could be made by a simple amalgamation and table concentration process. At depth, however, the proportion of free gold in the ore decreased, and a greater amount was intimately associated with sulphide minerals, mostly pyrite. Fine grinding and cyanidation was tried for some years, but eventually it was found that the most satisfactory method of treatment consisted of flotation, followed by cyanidation of the flotation concentrate reground to 325 mesh to release the values.
A plant with a capacity of 2,000 tons per day is now in successful operation. The ore is ground to 65 mesh, 55% being minus 200 mesh, in five tube mills, each in circuit with a “ Sub-A” Cell and a Dorr Classifier. A xanthate mixture is added with some aerofloat to the mills, and 75% of the precious metals is recovered from the flotation cells by the method already described. The pulp overflowing the classifier is then floated with additional aerofloat in eight six-cell Denver “ Sub-A” Machines in parallel, the tailings from which are pumped to six similar machines where a final clean-up is made. No cleaning is practised; the whole of the concentrate from both series of machines is filtered, reground in cyanide solution, and then treated by an agitation process in the cyanide section of the plant. A total recovery of more than 97% of the gold and silver is made by flotation, while the combined flotation-cyanidation process gives an overall extraction of 95%. The installation is much more compact and its capital cost far less than one embodying fine grinding and cyaniding of the whole tonnage, and it can be run with lower operating, maintenance, and depreciation costs.
A somewhat similar procedure has been adopted. Here the gold is present in the ore in three forms:
- As free gold, which is recoverable on corduroy strakes and is soluble in cyanide solution,
- as gold tellurides, which are not readily soluble in cyanide solution unless roasted, and
- as gold intimately associated with pyrite, which again needs to be roasted to give a satisfactory recovery by cyanidation.
Dry grinding of the ore followed by roasting and agitation with cyanide gave a good extraction in a plant treating about 500 tons per day, but the running costs were very high. It was then found that modern flotation methods would enable 90% of the mineral content of the ore to be recovered in a concentrate amounting to less than 10% of the weight of ore treated, the concentrate being so high in sulphur that no external fuel was necessary to roast it. An installation with a capacity of 1,500 tons per day is now in operation on this basis.
Three-stage grinding in ball and tube mills is practised, the product delivered to the flotation section containing 95% of minus 200 mesh material. Between each stage are corduroy strakes on which free gold is recovered to the extent of 25% of the total gold content of the ore. Sodium aerofloat is added to the ball mills and thio-carbanilide to the tube mills. The discharge from the third grinding stage is pumped to a surge tank, pine oil being fed to the pump intake and aerofloat to the tank. Flotation is then carried out, as shown below, in three rows of ten-cell “Sub-A” Machines, size No. 24, which comprise the roughing circuit. In the first cell of each machine, sometimes in the first two cells, a finished concentrate is made. The froth from the remaining cells, which contains a good deal of gangue slime, is sent to a ten-cell M.S. Machine (24-in. impellers) and cleaned with no further reagents except a small quantity of sodium silicate to depress the gangue. The cleaner tailing is returned to the tube mill circuit and the rougher tailing is discarded; the combined concentrate, amounting to between 6 and 7% of the tonnage milled, is dried, roasted, and cyanided. An overall extraction of more than 90% of the precious metals is made by the combined process. The cost of treating 500 tons of ore per day by the old method was about 16s. per ton. The new installation occupies no more space than the old one and can take 1,500 tons per day at a cost of 6s. per ton.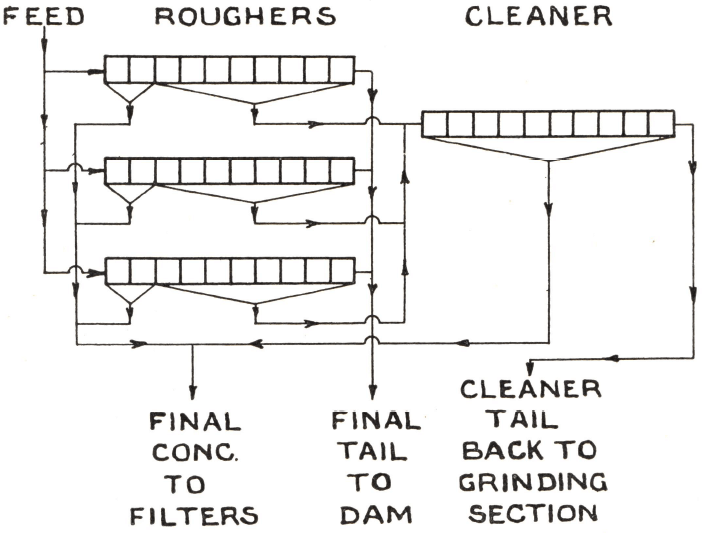
The two installations just described are typical of the way in which flotation is being applied in the metallurgy of gold ores. Although it is scarcely possible at present to point to a standard mode of procedure in their treatment, the fact has been established that flotation is of definite value as a means of reducing costs by concentrating into a small bulk gold-bearing minerals which require fine grinding or roasting before being cyanided. Its application to the treatment of free-milling ores, however, has not yet been proved in the same way, in spite of the undoubted simplicity and low capital cost shown by a flotation-cyanide plant as compared with an installation for cyaniding the whole tonnage milled, whether the latter involves fine grinding and cyaniding in one circuit, or coarse grinding and classification into sand and slime with a separate circuit for each product.
Whatever the flow sheet of any combined process for extracting the precious metals, the flotation section is usually comparatively simple. The aim is not so much to obtain a clean concentrate, although this can often be done with xanthate and aerofloat reagents, as to make the highest possible recovery of all the minerals with which the gold and silver are associated. Sometimes the concentrate from every cell is sent to the cyanide section without being cleaned; sometimes the froth from the last few cells is returned to the head of the machine, as in circuit 1. If cleaning is necessary, it is unlikely that more than one stage will be required, and circuit 2 will usually prove satisfactory, although some modification, such as the circuit shown above, may occasionally be necessary.
Mechanically agitated machines have been found, on the whole, to give the best results with gold ores. The reason appears to be that free gold and some gold-bearing iron sulphides tend to float rather slowly, and vigorous agitation speeds up the flotation rate by intensifying their degree of flocculation. The “ Sub-A” Machine is employed more frequently than any other for this class of work.
The reagent combination usually consists of aerofloat in conjunction with a dithiophosphate and a xanthate, with the addition of pine oil, if required, to increase the volume of froth. Sodium diethyl-dithiophosphate, is one of the most successful promoters for the flotation of free gold, while their Reagent “ 301 ”, a higher xanthate mixture, has been found specially useful for gold-bearing pyrite; sodium aerofloat and amyl xanthate are sometimes employed in place of or in conjunction with them. Flotation is generally carried out in a pulp with a pH value of 7 .0-7 .5, soda ash being the best reagent to employ for regulating the alkalinity, should one be necessary, since lime has a decided tendency to depress free gold and pyrite. The addition of sodium silicate to deflocculate gangue slime should be made with caution, as too much of it may depress the gold as well. If a deflocculator is required, starch, added up to 0.2 lb. per ton, is preferable to sodium silicate. Whichever reagent is used, it is better to introduce it into the cleaning circuit if possible, because any excess entering the machine through occasional faulty control is likely to cause a lower gold loss there than in the roughing circuit. The addition of copper sulphate up to 0.25 lb. per ton is sometimes found useful in speeding up flotation.
The normal range of the reagent consumption for ores containing free gold and gold-bearing iron sulphides is approximately as follows:

