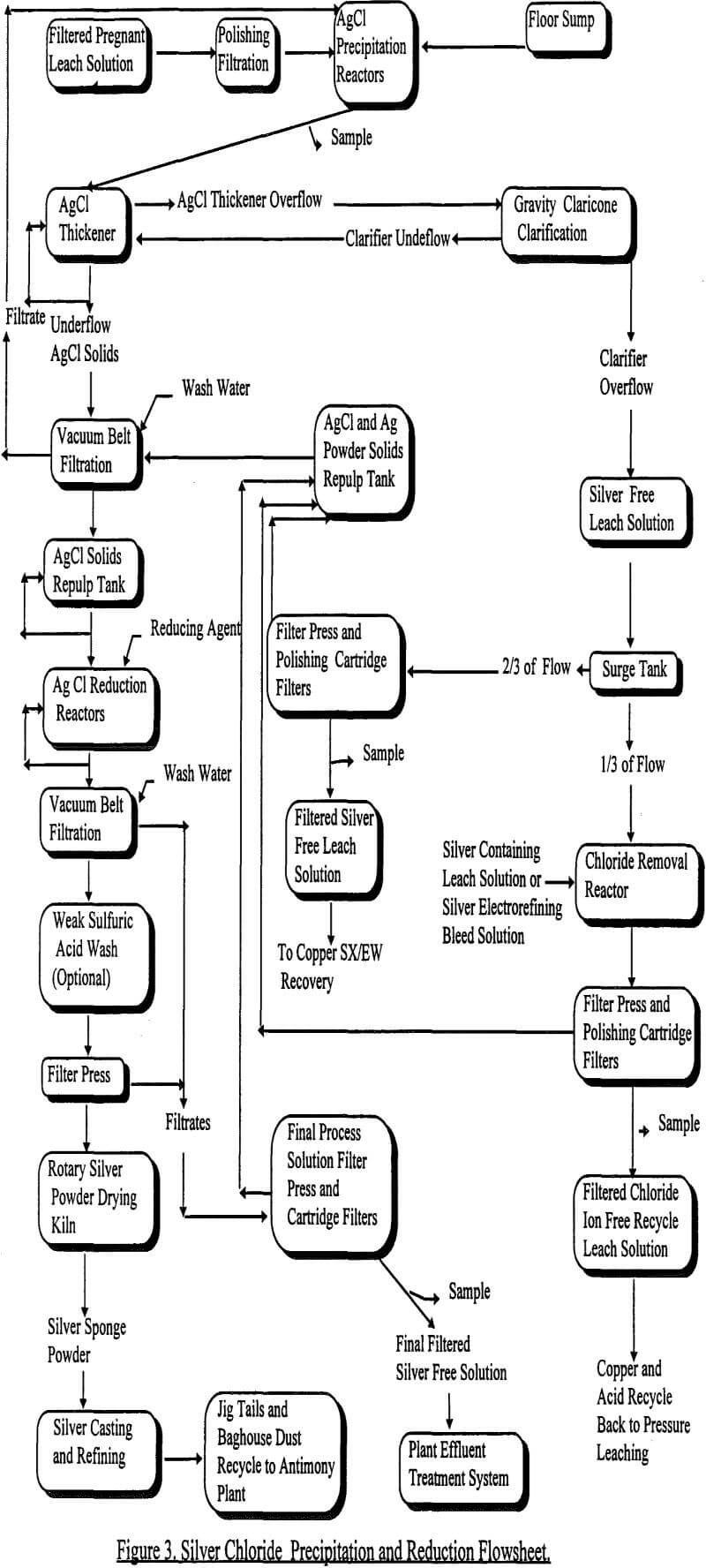
The unique insolubility of silver chloride makes it possible to readily achieve a distinctive separation of silver from a mixed metal ion acidic solution. Using chloride precipitation with NaCl solution, the plant operation selectively removes all the silver from the pressure leach solution. The chemistry is quite straightforward and effective. As with most processes, it can be achieved in a batch mode on a lab scale where tight process control and materials handling are not significant parameters. Unfortunately, the facility did not economically lend itself to small batch modes of precipitation. Thus, many of the challenges involved in the startup of this novel plant revolved around the continuous mode of precipitating silver chloride.
By conventional screening, thickening and pressure filtration, the pregnant solutions at Sunshine emanating from the pressure leach are first separated from the solid residues. This produces a clear, emerald green, “pregnant” solution containing less than 10 ppm solids. The pressure leach residue contains insoluble lead and antimony compounds that would contaminate the silver downstream if not separated from the solution before silver chloride precipitation. The clarified solution flows through two continuous stirred tank reactors (i.e. CSTRs) in series at up to 11.5 m³/hr. These tanks are constructed from fiberglass and each has a capacity of 1400 liters. The solutions are agitated with 1.5 kilowatt single rubber covered axial flow impellers. This configuration allows an approximate residence time of 15 minutes.
A premixed 10% NaCl solution flows by gravity into each tank and the flow is metered by plastic valves actuated by solenoids. This reaction, illustrated previously in equation 16, is quite rapid. From a chemical standpoint, there is no problem with this method. From a process control standpoint, it is a challenge. The first problem is to measure the extent of the reaction. Next, this information must be efficiently translated to control the NaCl flow. Both of these problems were solved by progressions of new and innovative technology.
Early on, it was decided to monitor this distinct reaction through use of ion-selective electrodes to measure concentrations of excess chloride ion. Because silver chloride is so insoluble, any excess dissolved silver will remove essentially all the chloride ion.
Conversely, once a slight excess of chloride ion is added, all the soluble silver is precipitated and the chloride ion concentration quickly increases. Less than 1 ppm of silver can be left in solution if the reaction is carefully controlled. By using ion-selective electrodes, it was found that excess chloride ion was easily measurable and that the point where all the silver had been recovered as AgCl was detectable.
In particular, laboratory chloride ion-selective electrodes were first tried to control the industrial process. One of the characteristics of this type of probe is that it is not stable. As it ages over a period of hours, the set reading of any particular chloride concentration changes and the sensitivity decreases. This is not a fatal flaw, however, and it was overcome in plant operations by operator monitoring techniques.
The loss of sensitivity in the commercial probes can be tolerated up to the point where the response is much too slow for the control system to respond. Then, the probe has to be changed. The 15 minute residence time designed into the reactor has proven to be long enough to tolerate this slow response. Probes will typically last from one to several days, and they are changed as required by the operators.
As far as a control scheme is concerned, the initial process control loop was controlled by oxidation reduction potential (i.e. ORP) process millivolt control meters. While this simple process control was effective, it did produce swings in residual chloride concentration of plus or minus 100 ppm. Thus, a set point of over 100 ppm excess chloride ion had to be maintained to assure the silver was completely recovered from solution. At times, the operators would resort to even higher excess chloride concentrations to be confident of the silver removal. However, the amount of residual chloride ion left in solution was still a plant process problem. As mentioned in the brief synopsis of the plant process, the recycle of one third of the silver solution for acid and copper recycle is a crucial step. This stream requires removal of all chloride ion before recycling back to the leach step. As this is accomplished with the production of more AgCl precipitate, it can pose a filtering problem if the stream to be treated contains a large amount of excess chloride ion.
The first difficulty in handling silver chloride is the propensity it has to pack together and form “prills”. These are as large as 3 cm in diameter and provide great resistance to pumping or mixing. Long residence times with low mixing speeds combined with high percent solids slurries seem to promote this phenomenon. “Seeding” by any other solids or smaller prills present can also aid in their development. During the startup phase, prill formation was common throughout the processing scheme. Eventually this was remedied by providing larger 15 cm diameter overflow outlets and a tank bottom drain in the CSTRs. To these bottom drains, diaphragm pumps on a timing setup are attached to occasionally pump out any buildup of solids. This stops any “seeding” caused by smaller prill formation and minimizes any substantial buildup of solids.
In the processing system, the characteristic of silver chloride to pack and agglomerate appeared everywhere. In the thickener, where a set of rakes had been installed, the silver chloride eventually packed into an enormous, slowly revolving ball. To solve the problem the thickener rakes were removed. Now, the operators stir the thickener solids bed by hand several times per shift to keep the solids moving through the pumping system. Additionally, throughout the piping system, plugging occurred at joints and elbows. To alleviate these, larger diameter pipes were installed and all unnecessary bends were removed.
The second persistent problem met in separation is settling and filtering the silver chloride. While it will settle rapidly, a clarified solution is not always produced. This can significantly impact the downstream filterability of the solution. Surprisingly, it was found that while flocculants had no effect on the clarity of the solution, adequate agitation did. By thoroughly agitating the slurry after precipitation is complete, it was found that a sparkling, clear solution was obtained after settling. This process of adequate agitation was accomplished in the existing system by performing the majority of the AgCl precipitation in the first CSTR. This allowed the second CSTR to act as an agitation conditioning tank before settling. This method of precipitation clarified the solutions to the point that the additional gravity clarifier, shown in the Figure 3 flowsheet, became a redundant, but useful, process step when AgCl solids happen to overflow the thickener.

Finally, the last challenge posed to this system was in the pumping and washing of the silver chloride solids coming from the thickener underflow. Like the precipitation and thickening system, this part of the operation was plagued initially by silver chloride agglomeration and packing. Initially, a 2.5 cm pipeline carrying a nominally low flow of 0.2 to 0.5 m³/hr fed the belt filter from the thickener underflow. The flow was unsuccessfully controlled by adjusting the pump speed and by throttling a diaphragm valve. Because of the small diameter pipe and the restriction at the control valve, this low flow proved impossible to maintain due to line plugging. To alleviate this problem, a recirculating slurry system was installed analogous to those commonly used in handling lime slurries. Here larger 3.75 cm pipes were used, the flow was increased to 3.5 m³/hr, and the diaphragm valves were replaced with full port air actuated rubber sleeve pinch valves. In this manner, a bleed of 0.2 to 0.5 m³/hr of silver chloride slurry flow from the recirculating line was established to the belt filter. To accomplish this, the pinch valves operated in the full open or full closed position to allow accumulated solids to pass through easily. The resulting flow was controlled by asymmetric timers that allowed for tight control of the slurry flow on to the belt filter. To assure a good discharge of slurry, a back pressure valve is actuated in the reverse mode of the filter feed valve.
From the bottom of the thickener, silver chloride solids are pumped to a horizontal belt filter. Here, entrained leach solution is filtered away. The filter cake passes under a series of wash trays to clean the solids. The solids then fall off the belt into a tank where they are repulped with water. From here they are pumped over to the silver chloride reduction circuit where silver sponge is produced. By referring to Figure 3, this sequence of events can be traced.
The resultant silver free filtrate from the silver chloride processing steps is now ready for further processing. Two thirds of this solution is treated for its copper content by solvent extraction and electrowinning. To insure no silver chloride particles are lost, a final polishing filtration is performed. This is accomplished by a small in-line filter press followed by filter cartridges. Periodically, the solids in the press are removed and sent to the silver chloride reduction circuit. Initially, this stream consistently ran 20 ppm silver. It was surmised that this was soluble silver and not escaping silver chloride particles. However, lab testing indicated with proper filtration less than 1 ppm of silver could be left in solution. In the plant, this was attained by switching from wound filter cartridges to pleated polypropylene cartridges. An estimated capture of an extra 510 kg per year of silver that would have been lost resulted from this switch. Additionally, the pleated cartridges had the advantage of being cleaned for reuse. This minimized the accumulation of wound cartridges high in silver value.
The other one third of the silver free solution is processed for acid recycle to the pressure leach area. This stream has been alluded to in previous passages. As it makes up a considerable portion of the flow through the plant, it is an important consideration in the overall operation. Its chemistry is exactly the same as the initial pregnant leach solution precipitation operation illustrated in equation 16. Again, a silver chloride precipitate is produced from the solution. However, in this case, excess chloride ion is being reacted with a silver containing stream to produce a chloride ion free stream. Initially, this reaction was attempted in a pipeline with an in-line mixer. A metering pump provided silver solution for the reaction just ahead of the in-line mixer. An in-line laboratory ion selective electrode was used to control silver solution addition. In theory, silver chloride would be formed in the pipeline to cleanse the solution of chloride ion. A series of bag filters would then remove the silver chloride solids from the slurry. This configuration proved impossible to control due to plugged lines, pump problems, electrode failure and filtering difficulties. Presently, this recycle task is handled in a series of CSTRs. In this case, 3 small overflow vessels are used. Solution stripped of silver and containing chloride ion is pumped through these reactors for treatment. A stream of pregnant leach solution is added to the vessels as a source of silver ion for the reaction. Again, the reaction is monitored by the low cost ion-selective electrodes made on site. As the flow situation is not as severe in this circuit, simple on-off setpoint ORP meters are used for process control. After the reaction is complete, the solid silver chloride produced is filtered by a filter press followed by a pleated cartridge filter. The resulting solution, which has an excess of 200 ppm silver with essentially no chloride ion is pumped back to the pressure leach area as a source of sulfuric acid. Periodically, the filter press containing the silver chloride solids is manually cleaned and these solids are eventually sent to the silver reduction circuit.
