Table of Contents
- Cyanidation-Barite Flotation
- Cyanidation Tests
- Barite Flotation of Cyanidation Residues
- Salt Roasting-Cvanidation-Barite Flotation
- Salt Roasting-Cyanidation Tests
- Barite Flotation of Salt Roasted-Cyanidation Residues
- Barite Flotation-Cyanidation and Flotation-Salt Roasting-Cyanidation
- Barite Flotation
- Cyanidation and Salt Roast-Cyanidation Tests on Composited Flotation Tailings
The Calico mining district in California contains extensive deposits of low-grade silver ore. The deposits cover a large area and constitute an important silver reserve if difficult concentration problems can be solved. The deposits also contain barite, which, if recovered as a byproduct during treatment of the silver ores, might contribute to the economic returns from the ore. Previous beneficiation work on ores from the Calico district was reported by Val Kudryk who tried normal and fine grinding followed by cyanidation, salt roasting and cyanidation, oxidizing or reducing roasts and cyanidation, chloride volatilization, and SO2 leaching followed by cyanidation. Kudryk’s best results were obtained (1) by a salt roasting-cyanidation procedure that yielded more than 80 percent recovery of the silver in the ores or (2) by a fine-grinding-cyanidation treatment of unroasted ores with 60 to 65 percent recovery. In spite of its higher silver recovery, the salt roasting-cyanidation process is more costly and does not permit production of a barite byproduct; thus, the fine-grinding-cyanidation process may be competitive.
The objective of the Bureau’s research, which was part of the silver and gold program of the Bureau of Mines, was to investigate alternative methods for beneficiating the Calico ores to recover the silver and barite.
Source and Nature of Samples
The Calico mining district is located in San Bernardino County, Calif., about 10 miles east of Barstow in the central part of the Mojave Desert. Mining operations in the Calico district started in 1882 and continued until 1896 when the low price of silver made operations unprofitable. During the period of 1882 to 1896, a total of between $13 and $20 million in silver was produced from a number of high-grade, shallow deposits. There was only sporadic activity in the area from 1896 until about 1963, when the mining industry again became interested in the district because of an increase in the price of silver from 91 cents per ounce in 1961 to $1.29 per ounce in 1963. Furthermore, there was the possibility of additional silver price increases due to expanding world industrial and monetary demand. Several companies initiated exploration programs to determine whether there were additional, large deposits of low-grade ore in the formations that had originally been mined for rich, surface pockets of silver ore.
All of the samples of Calico ore studied at the Salt Lake City Metallurgy Research Center, Bureau of Mines, were obtained from rotary drill cuttings and were supplied by the companies doing exploration work in the area. Although six samples were studied over a period of several years, only four, which weighed 300 to 400 pounds each, were large enough for thorough metallurgical testing.
Representative portions of the four bulk samples were submitted for chemical analysis, mineralogical study, and metallurgical testing. Chemical analyses of the four samples are given in table 1.
Microscopic examination of polished briquets made from magnetic concentrates and from heavy mineral concentrates from elutriation did not reveal any discrete silver minerals. However, X-ray microprobe examination of a silver concentrate obtained by xanthate flotation showed native silver in particle sizes of 3 to 4 microns. The native silver was associated with similarly sized chalcopyrite grains. No appreciable quantities of silver sulfide or oxide minerals were indicated by the microprobe examination.
Barite was a common constituent of all of the samples and occurred as tabular cleavage fragments that were generally free of inclusions or coatings.
The major gangue minerals were quartz and potassium feldspar. The quartz occurred both as cryptocrystalline chalcedony and anhedral to euhedral crystalline grains. The feldspar occurred as altered grains that contained abundant inclusions of clay minerals.
Other minerals present in lesser amounts in the samples include sericite, biotite, chlorite, calcite, pyrite, and magnetite. Zircon was found in small amounts in samples C and D; sphene in sample C; and rutile, epidote, and an amphibole in sample D. Mineralogical examination failed to reveal any specific differences that might explain variations in how the samples responded to treatment.
Objectives of Research
The principal objective of the research presented in this report was to develop alternative methods of beneficiating the low-grade ores in the Calico district. Silver is the most valuable mineral in Calico ores; thus, most of the research effort was directed toward recovering the silver. Since it was possible that a market could be developed for the barite present in the Calico ores, studies also were made to recover the barite as a salable byproduct. A secondary objective was to obtain (1) grinding, cyanidation, and flotation data that would define the optimum reagent combination and (2) operating conditions that would be required for maximum silver and barite recovery.
Laboratory Research
In the studies made on the Calico ore samples, a variety of methods was investigated to recover the silver, including segregation and flotation of salt-roasted ore, xanthate flotation of ore, magnetic separation of finely ground and reduced ore, and cyanidation of both unroasted ore and salt-roasted ore.
Flotation was the only method used to recover the barite. During the barite flotation research, oleic acid, a petroleum sulfonate, and sodium cetyl sulfate (SCS) were investigated as barite collectors. Oleic acid was the least selective of the collectors, whereas SCS was the most selective and gave the highest barite recovery at the highest barite grade.
Preliminary Investigations
Preliminary research was designed to determine the most effective methods of recovering the silver and barite. Segregation-flotation, xanthate flotation, and magnetic separation were investigated as methods of recovering the silver in sample A. Because none of these methods resulted in recovery of more than 38 percent of the silver, they were not used on the later Calico samples. Cyanidation of ground, unroasted ore and cyanidation of salt-roasted ore offered the opportunity for greater silver recovery, and these methods were used on subsequent samples.
In the initial barite flotation research, oleic acid was used to float the barite. Recovery of barite at specification grade (plus 92 percent barite) averaged about 60 percent. Use of American Cyanamid AERO Promotor 825, an anionic petroleum sulfonate, and sodium silicate in an alkaline circuit improved both selectivity and barite recovery, which was increased to a range of 70 to 75 percent. Substitution of SCS for AERO 825 further improved selectivity, and barite recovery was increased to 85 to 90 percent.
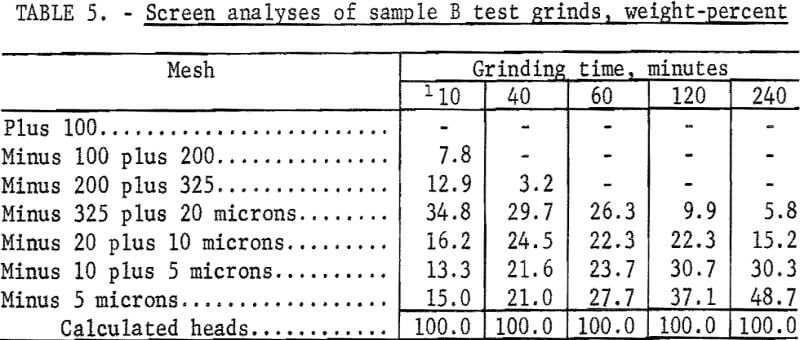
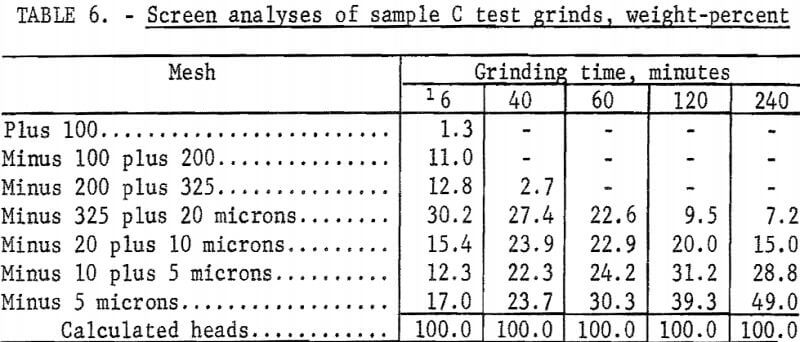
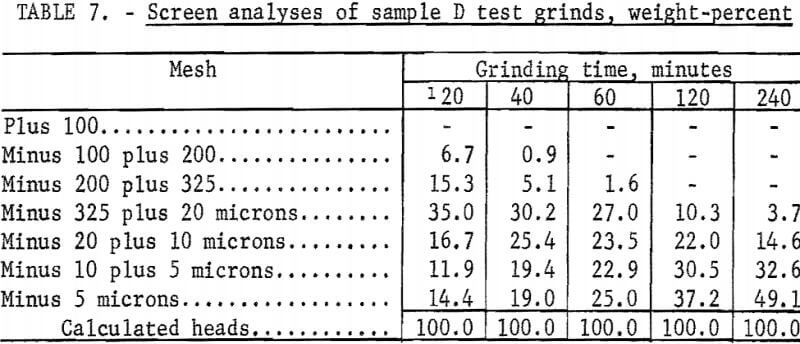
The Calico ore samples, as received, differed in mesh size and grindability, and thus required different grinding times to achieve the same grind. The 4-minute grind used on sample A to obtain the best recovery of barite by flotation is referred to as the standard grind for sample A; the amount of minus 20-micron material as shown in a screen analysis was used as a guide to obtain similar standard grinds for the other Calico samples. Screen analyses of standard grinds (10, 6, and 20 minutes for samples B, C, and D, respectively) for each of the samples are shown in table 2. Subscreen sizing was done by water sedimentation.
Laboratory batch studies were used to investigate three methods of concentrating the silver and barite in the Calico ores. These methods were
(1) cyanidation followed by flotation of barite from the cyanide residue, (2) salt roasting-cyanidation followed by barite flotation from the residue, and (3) barite flotation followed by cyanidation or salt roasting-cyanidation on the flotation tailing. Results of these studies are described in the following sections.
Cyanidation-Barite Flotation
A series of cyanidation-barite flotation tests was made on each of the samples to determine whether ultrafine grinding would improve silver recovery and also to investigate the effect of fine grinding on barite recovery from the cyanidation residues.
Cyanidation Tests
Cyanidation tests were made using the following procedure: A 500-gram charge of minus 10-mesh ore was ground at 50 percent solids in an iron ball mill with a 10,000-gram ball charge. Salt Lake City tapwater, which contains a total of 75 to 100 parts per million of calcium and magnesium, was used. Before cyanidation, all pulps ground for 60 minutes or longer were passed through a Frantz ferrofilter to remove grinding iron, which interferes with cyanidation. Finely divided metallic iron is reported by Hamilton to interfere with cyanidation by slowly reacting with and destroying cyanide. Research on the Calico ores showed that as grinding time approached 240 minutes, little silver was dissolved from 0.2 percent sodium cyanide pulps that contained the grinding iron, and cyanide consumption increased to more than 20 pounds per ton of ore.
The magnetic product from the ferrofilter was cleaned in a Davis tube magnetic separator, dried, weighed, and assayed for silver. The ground pulp was filtered, transferred to a 4-liter beaker, and agitated 24 hours with 1 liter of saturated lime water (0.1 percent lime) that contained 4 grams sodium cyanide (0.4 percent). When the leaching period was completed, the pulp was weighed and filtered. The residue was washed with 300 milliliters of tapwater before being removed to a 2-liter beaker for the first of two agitation-washing-filtration steps. Each washing step consisted of 4 minutes of agitation with approximately 1,000 milliliters of tapwater. The residue was dried, weighed, and sampled. Samples of the residue, the pregnant solution, and the wash solution were analyzed for silver by atomic absorption spectrophotometry. The residue was saved for barite flotation. Cyanide and lime consumption increased as the length of grind increased. Cyanide consumption was about 1.5 pounds of sodium cyanide for the coarsest grind and 3.5 pounds per ton for the finest grinds. Lime consumption ranged from 3 to 4 pounds per ton. Table 3 shows the results obtained by cyanidation treatment of Calico ore after grinding for periods up to 240 minutes and removing grinding iron from those ground 60 minutes or more. Tables 2 and 4-7 give screen analyses for the various grinds shown in table 3.
The silver recovery data in table 3 clearly show the refractory condition of the silver in the Calico samples that resists extraction despite ultrafine grinding. Only sample D is moderately amenable to the recovery of silver by the cyanide process at practical grinding levels. Silver recovery at this grind was 60.0 percent, a recovery not approached in the other samples unless 120- or 240-minute grinds were used. The screen analyses in tables 4-7 show that grinds of 40 minutes were virtually minus 325 mesh, whereas grinds of 240 minutes, considered only of academic interest, averaged about 80 percent minus 10 microns.
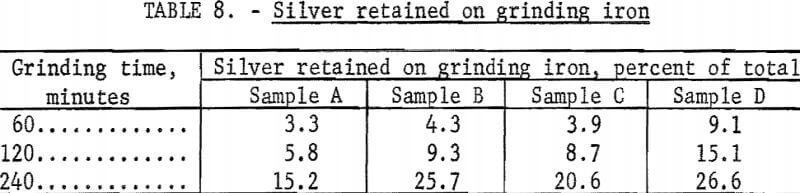
As previously mentioned, grinding iron interfered with cyanidation and was removed from the more finely ground pulps before cyanidation as a magnetic product that represented 3 to 9 percent of the weight of ore. The silver associated with the iron represented an appreciable portion of the total silver, particularly in the finest grinds. Table 8 shows the silver retained with grinding iron in samples ground 60 minutes or more. An effort was made to recover the silver from the grinding iron by cyanidation. Approximately 85 percent of the silver was recovered by cyaniding in a 0.2-percent sodium cyanide, 0.1-percent lime solution at only 10 percent solids for 24 hours. The treatment of the grinding iron product by cyanidation required the consumption of an additional 1.4 pounds of sodium cyanide and 0.6 pound of lime per ton of original ore feed. Tests to upgrade the material by leaching with hydrochloric acid, sodium chloride and sulfuric acid, and ammonium hydroxide were unsuccessful.
Barite Flotation of Cyanidation Residues
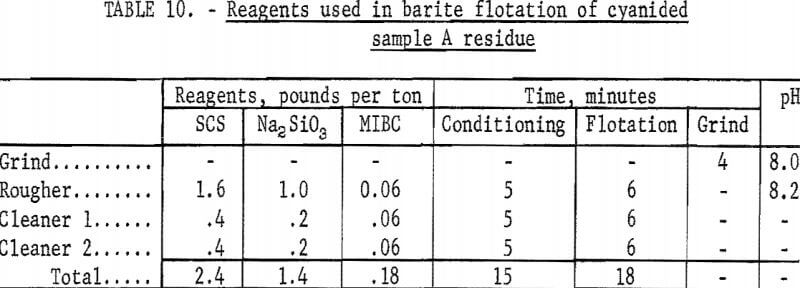
The best barite flotation results were obtained by the following procedure: The residue from cyanidation was transferred to a Fagergren laboratory flotation cell equipped with a glass bowl, stainless steel impeller and stator, motor controller, and tachometer. The pulp was diluted to 25 percent solids with tapwater and agitated for 4 minutes at 2,000 rpm without reagents to insure complete separation of all particles. Sodium silicate and SCS were added and conditioned together at 2,000 rpm for 4 minutes at a pH of 8.2 to 8.5. A frother, methyl isobutyl carbinol (MIBC), was conditioned in the pulp for 1 minute before the barite rougher froth was collected. No frother was required to float the barite in sample D residues. The rougher froth was cleaned twice at 2,000 rpm using small amounts of SCS and sodium silicate in each cleaning. The separate products were filtered, dried, weighed, and analyzed. Tables 9-10 show the results of a typical barite flotation test and the amount of reagents used, respectively. Table 11 shows concentrate grade and recovery obtained by floating barite from the cyanidation residues of Calico ore samples.
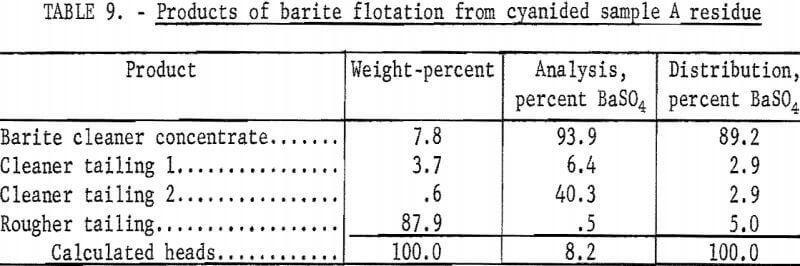
The data in table 11 show that cyanidation treatment of the Calico ore samples does not affect the flotability of the barite. Ultrafine grinding, however, has a marked effect on all samples, particularly on samples B and C, from which only 47.9 and 34.0 percent of the barite, respectively, could be floated in a specification-grade product after a 240-minute grind. Flotation in the finer sizes, especially in the pulps ground 120 and 240 minutes, were characterized by voluminous and tough froths, the need for an extra cleaning step, and a higher barite tailing assay.
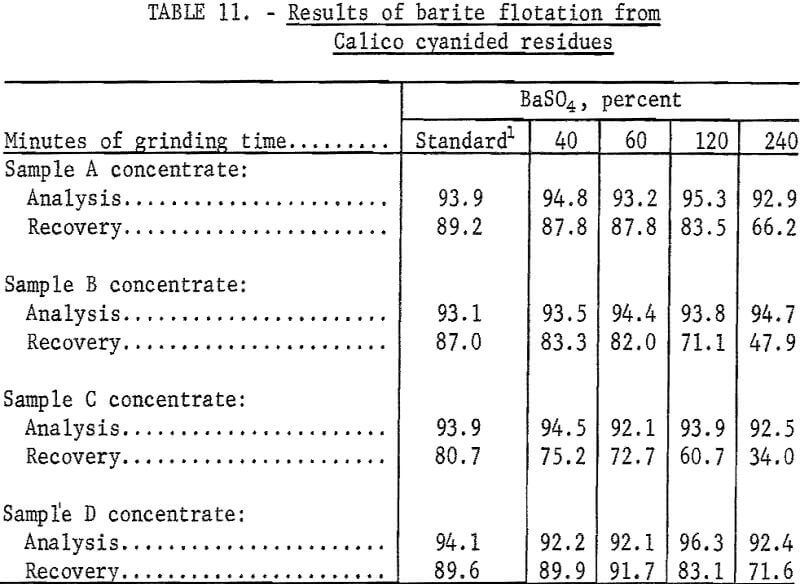
To determine barite marketability, specific gravity determinations and screen analyses were made on the barite concentrates obtained from cyanide residues that had received the standard grinds. Specific gravities of these products ranged from 4.2 to 4.3, which met the specific gravity specification for barite used in drilling mud. The barite products from samples A, B, and C did not meet the size specification of 90 to 95 percent finer than 325 mesh, having only 78 to 86 percent minus 325 mesh. Sample D barite concentrate was 99 percent minus 325 mesh. Barite products from 40-minute grinds were 100 percent minus 325 mesh. Barite specifications are given in the appendix.
Salt Roasting-Cvanidation-Barite Flotation
The refractory Calico ore samples are not very amenable to the recovery of silver by the cyanide process despite grinding to 80 percent minus 10 microns. Salt roasting followed by cyanidation was investigated as a means of improving the silver recovery. The residues from cyanidation were treated by flotation to recover the contained barite.
Salt Roasting-Cyanidation Tests
Development of a salt-roasting procedure for the Calico ore samples required determination of optimums for the amount of salt, the particle size of the sample, the temperature of the roast, the length of time in the furnace, and the grind of the roasted material before cyanidation.
The optimum amount of salt was determined from a series of tests in which salt additions were varied from 1 to 10 percent. The results of these tests showed that the best silver recovery was obtained with 6 percent sodium chloride.
Most of the test work to determine optimum particle size for material being salt roasted was done on samples that had been finely ground on the assumption that better chlorination would occur with small particles rather than large ones. However, a series of tests with material as received, which was minus 10 mesh for samples A, B, and C and minus ¼ inch for sample D, proved the assumption to be incorrect. Silver recovery from the as-received samples was equal to that obtained from material finely ground before the salt roast.
The range of temperatures investigated was from 700° to 900° C. The silver recovered by cyanidation after roasting samples A, B, and C at 750° and 800° C was noticeably less than the silver recovered at 850° and 900° C. However, the silver recovered at 900° C was only 3 to 4 percent more than that recovered at 850° C. The optimum temperature for sample D was 850° C; silver recovery was lower at both higher and lower temperatures. Based on a comparison of the assay head with the calculated test heads, from 6 to 12 percent of the silver was volatilized during the salt roast. The quantity of volatilized silver increased with the roasting temperature and furnace time.
Only two furnace times were investigated. Slightly higher silver recoveries were obtained with the longer residence time in the furnace. For this reason, the final series of salt roasting-cyanidation-barite flotation tests used the longer time, as shown in table 12.
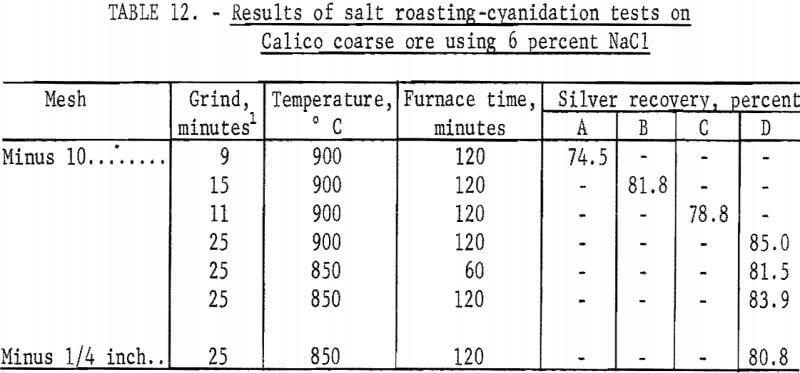
In the study to determine optimum regrinding times for the Calico samples, most of the tests were made on material ground to flotation size before roasting employing the standard grind for each sample. Roasts made from such material were reground for as little as 5 minutes or as much as 60 minutes. As previously mentioned, the longer grinds required magnetic separation to remove grinding iron that hindered cyanidation and contained some of the silver in the sample. Regrinds for the roasting tests on coarse Calico material were the standard grinds of each sample plus 5 minutes (table 12).
Salt roasting-cyanidation tests were made using the following procedure: A charge of 500 grams of minus 10-mesh ore was thoroughly mixed with 6 percent minus 100-mesh salt, placed in a fire clay dish, and charged to the furnace, which had been preheated to the selected temperature. The charge was rabbled for 15 seconds every 10 minutes. At the end of the roast, the charge was removed from the furnace and air-cooled. The roasted material was ground and cyanided as previously described. Consumption of cyanide and lime averaged 1.5 and 3.0 pounds per ton, respectively. The residues were saved for flotation to recover the barite. The results obtained by salt roasting and cyaniding Calico coarse ore are shown in table 12. Recoveries are based on calculated heads, assuming that, in practice, more than 90 percent of the volatile silver would be recovered in a fume-retrieval system and leached with the roasted ore.
The data in table 12 show that salt roasting of coarse ore followed by cyanidation resulted in silver recoveries that were approximately 25 percent higher than those obtained by cyanidation alone with ultrafine grinding; the data in table 12 do not include silver recoverable from grinding iron removed prior to cyanidation.
Barite Flotation of Salt Roasted-Cyanidation Residues
The residues from the salt roasted-cyanidation tests were treated by froth flotation to recover the barite. As in the series of tests in which barite was floated from cyanidation residues, SCS, sodium silicate, and MIBC were used. Each rougher froth was cleaned three times in an attempt to produce specification-grade barite concentrates. The results of these tests are shown in table 13.
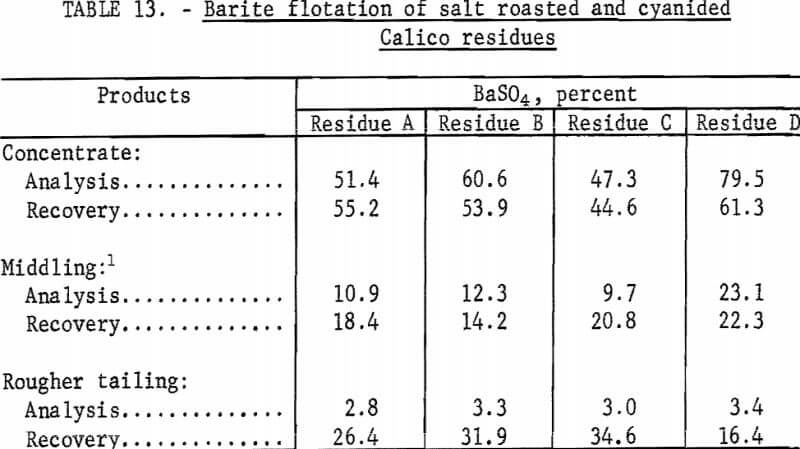
The results of barite flotation tests made on salt-roasted residues show that none could be treated to produce barite concentrates that meet minimum specifications.
Although the amount of collector used was doubled to improve the collection of barite and lower the content of the tailing, there was no improvement in results. Tripling the sodium silicate likewise did not make cleaner products or improve flotation results.
Tests were made to determine the reasons for the failure of any reagent combination to obtain satisfactory flotation of the barite. Calico ore roasted at 850° or 900° C for 1 or 2 hours, without any salt, behaved exactly as did the salt-roasted residues-tailings had high barite contents, and concentrates were low in barite.
Barite Flotation-Cyanidation and Flotation-Salt Roasting-Cyanidation
A series of tests was made in which barite was floated first and then the combined cleaner and rougher tailings were treated by either cyanidation or salt roasting-cyanidation to recover the silver. The procedure of salt roasting and cyanidation after barite flotation has the disadvantage of requiring a costly dewatering step of the barite flotation tailing to permit salt roasting.
Barite Flotation
Several similar flotation tests using standard grinds for flotation were made on each sample using SCS to float the barite. The previously described procedure was followed in all of the flotation tests. Barite flotation from untreated, natural ore required only one cleaning step to make a plus 92-percent barite concentrate, whereas two cleaning steps were required to recover the barite from cyanide residues. The flotation rougher tailing and cleaner tailing from each test were mathematically combined for reporting purposes. The flotation tailings from the several tests on each sample were composited by weight and saved for further cyanidation testing. The barite flotation results shown in table 14 are mathematical composites of the individual tests on each sample.
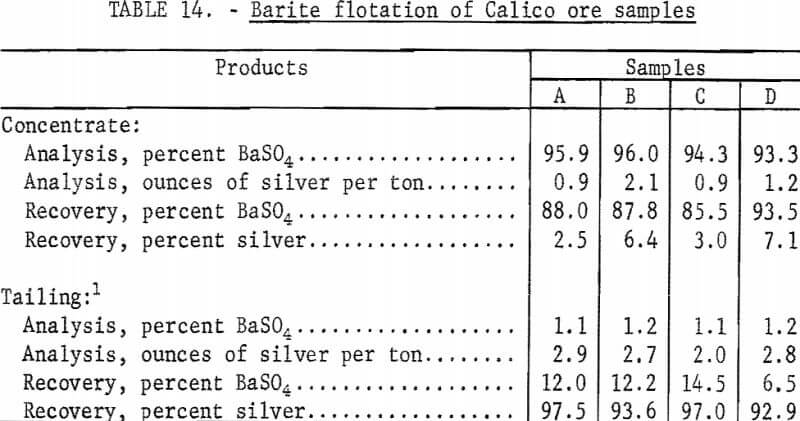
As shown by the data in table 14, barite is easily floated from untreated, natural Calico ore. The recovery of barite from the four samples ranged from 85 percent to 93 percent in concentrates that contained from 93 to 96 percent barite. Silver in the combined tailings ranged from 93 to 97 percent of the silver in the samples.
Cyanidation and Salt Roast-Cyanidation Tests on Composited Flotation Tailings
The series of tests to recover silver from the composited flotation tailings had two purposes. The first was to compare the results of cyanidation of the flotation tailing with cyanidation of natural or salt-roasted ore. The second was to compare the effectiveness of potassium chloride and lithium chloride with sodium chloride as chlorinating agents. The salt roasting and cyanidation procedures were the same as previously described with the exception that 100-gram charges were used instead of 500-gram charges. Regrinds of 5 and 60 minutes were used to investigate the effect of grinding on silver recovery before cyanidation. Cyanide consumption was about 2 pounds per ton. Lime consumption ranged from 3 to 4 pounds per ton. The results of this series of tests are shown in table 15. Silver recovery was calculated on an overall basis of the silver in the original sample.
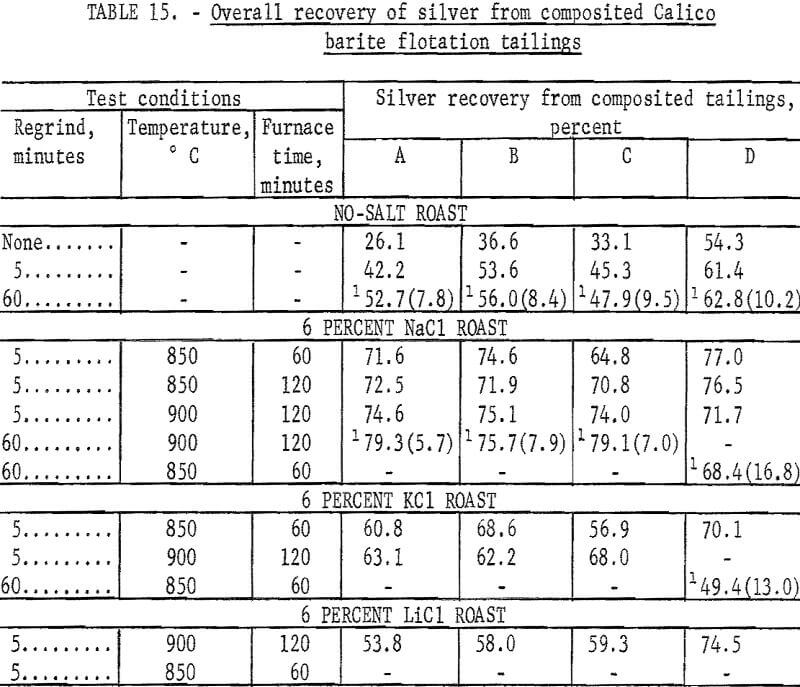
The data in table 15 show that silver recovery by cyanidation from unroasted or salt-roasted Calico flotation tailing is about the same as that from the cyanide treatment of natural or salt-roasted ore. Screen analyses of the 60-minute grinds used on the 100-gram samples show that this grind is comparable with the 240-minute grinds on 500-gram charges. Silver recoveries were higher when sodium chloride was used in roasting than when potassium chloride or lithium chloride was used.
Conclusions
- Cyanidation of Calico ore ground to virtually 100 percent minus 325 mesh recovered about 47 percent of the silver in three of the samples and 62 percent of the silver in the fourth sample. Barite flotation recoveries from the cyanidation residues ranged from 75 to 90 percent in plus 92 percent barite concentrates.
- The four samples differed widely in time of grinding required for equivalent fineness and in response to cyanidation. For example, sample D (2.6 ounces of silver) required 20 minutes of grinding to 100 mesh, and sample A (2.8 ounces of silver) required only 4 minutes, but 60 percent of the silver was cyanided from the D sample and only 29 percent from the softer A ore at 100-mesh size.
- Salt-roasting minus 10-mesh Calico ore samples before cyanidation increased silver recoveries to 75 to 85 percent. Barite could not be floated from the cyanidation residues in products that met minimum barite specifications.
- Flotation of the untreated ore recovered 85 to 93 percent of the barite in specification-grade barite concentrates. Cyanidation or salt roasting-cyanidation of the flotation tailing resulted in silver recoveries that were about the same as those obtained when silver was recovered first in the concentration procedure-either by cyanidation alone or salt roasting-cyanidation. However, the extra cost of dewatering and drying barite flotation tailing prior to salt roasting may be economically unattractive.
Appendix.-Barite Specifications
Chemical inertness, high density, and low cost make barite especially suitable for well-drilling muds. This use represents over 70 percent of the barium demand.
Specifications for barite vary according to its different uses. Material for drilling muds must be fine-ground, heavy, and chemically inert; consequently, barite for this purpose must be free of soluble salts, contain a minimum of 92 percent BaSO4, and have a specific gravity of 4.2. Several percent iron oxide is not objectionable. Also, 90 to 95 percent of the ground material must pass a 325-mesh screen.
Chemical manufacturing requires barite to meet more stringent specifications than for its other uses. Purity is the principal concern, and a maximum of 1 percent each of ferric oxide (Fe2O3) and strontium sulfate (SrSO4) and a trace only of fluorine usually are specified, with a minimum of 94 percent BaSO4. If the mineral is to be used for production of lithopone, a mixture of BaSO4 and zinc sulfide (ZnS), the SrSO4 content may be somewhat higher. Mesh size is important to chemical producers: If material is too fine, dust is lost; and if it is too coarse, mixing with carbonaceous material is poor. Most chemical industry consumers specify a size range of 4 to 20 mesh; however, some purchase lump and grind to their own needs.
Glass manufacturers usually require a 98-percent BaSO4 minimum with a maxima of 1.5 percent silicon dioxide (SiO2), 0.15 percent aluminum oxide (Al2O3), and 0.15 percent Fe2O3. The iron oxide is the most objectionable. The particle size generally preferred is a mixture ranging from 30 mesh down through 140 mesh. Material ground to 325 mesh is undesirable because it tends to ball up in the batch but can be used in times of short supply of the coarser material.
Specifications for barite used as a filler or in aggregates are less strict; in most instances size of grind is the most important.
