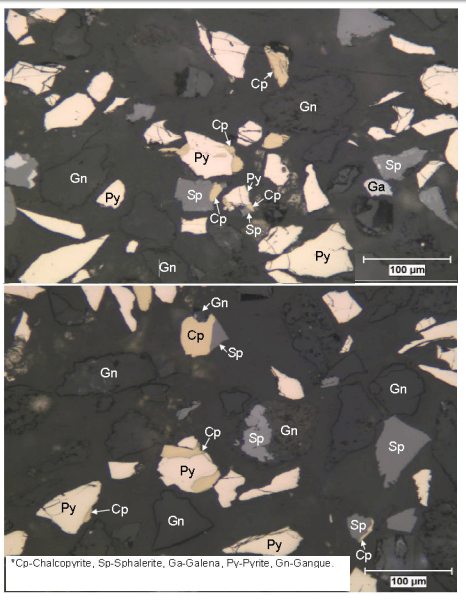
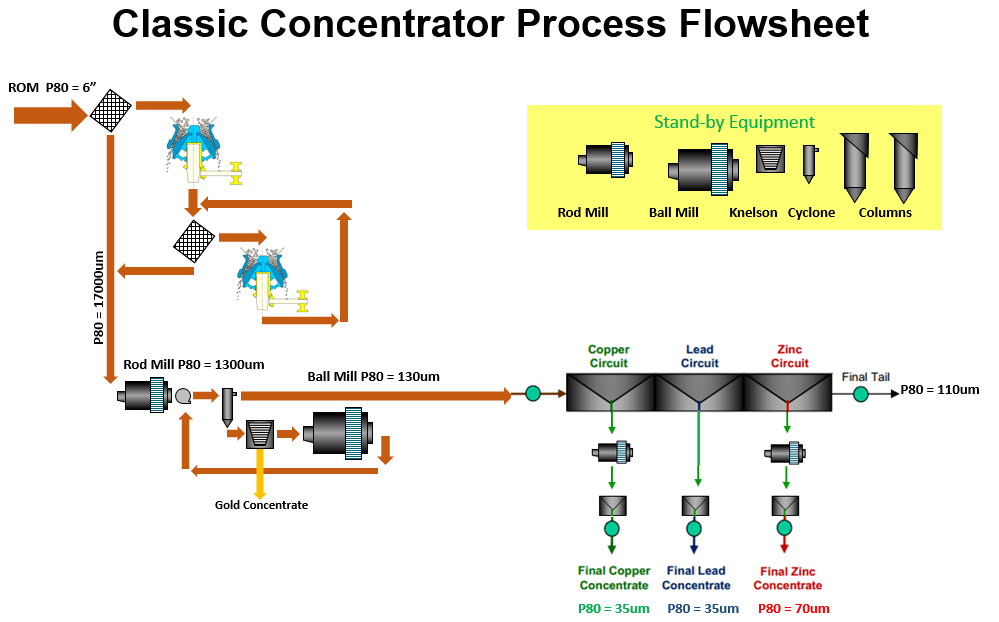
To understand froth flotation, first read that article and come back to read about sequential flotation of Copper, Lead and Zinc.
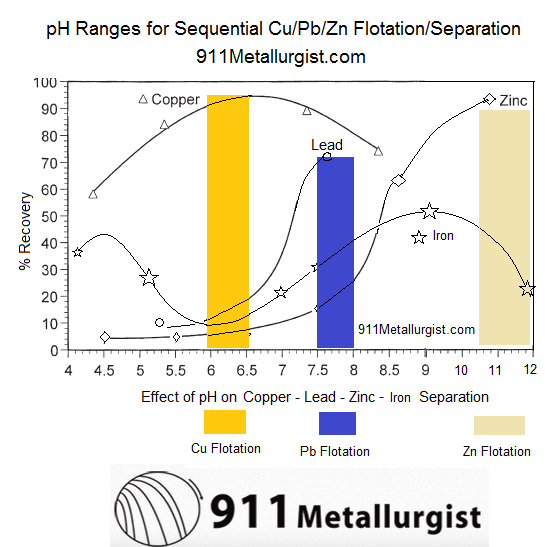
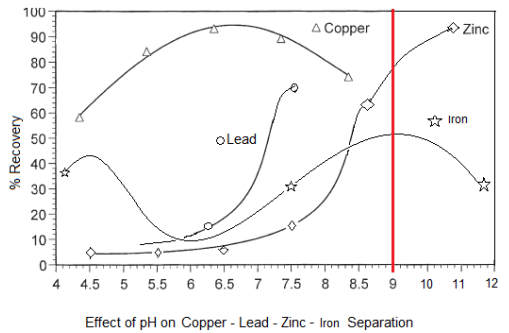
Given a baseline amount of frother and collector, these curves are the approximate/relative rate of flotation recovery of Copper (Chalcopyrite/Bornite/Chalcocite/Covellite/Tennantite/Enargite/Tetrahedrite /Freibergite ), Lead (Galena) and Zinc (Sphalerite) as well as iron (pyrite/arsenopyrite) minerals VS pH.
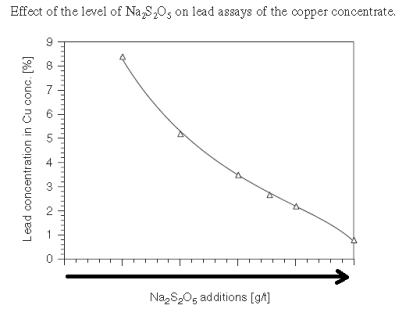
With a slurry’s natural pH is 9, it becomes clear Cu/Pb/Zn cannot be separated from each other nor from Fe. At pH 9 now, most of all metals would float. As shown here below, assuming Collector addition is Optimum, pH and MBS in the Copper Circuit drives Pb content in the Cu Conc.
Float Metals One-by-One Selectively: Since our objective is to sequentially float Copper, then Lead, than Zinc while rejecting Pyrite, let us explore how we can separate Cu/Pb/Zn from Fe:
- pH 6-6.5 is where Pb/Zn/Fe are least hydrophobic while Cu is most hydrophobic. Great pH to Recover Cu.
- pH 7.5-8 is where Zn/Fe are least hydrophobic while Pb (and Cu) is most hydrophobic. Great pH to Recover Pb.
- pH 10.5-12 is where Fe is least hydrophobic while Zn (and Cu/Pb) is most hydrophobic. Great pH to Recover Zn.
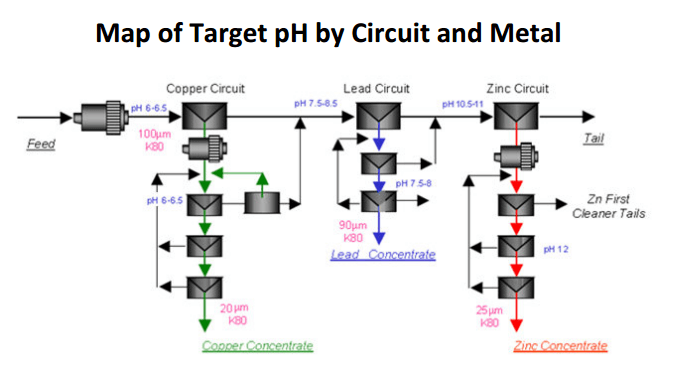 By modifying Flotation pH to create kinetic/speed differences between the metals and achieve sequential flotation and take full advantage of the hydrophobic nature of each mineral, it is best to first lower the pH and see about recovering copper. MBS is the pH modifier of choice to dropping pH into a zone where Cu is hydrophobic while all other metals are hydrophilic provided an opportunity.
By modifying Flotation pH to create kinetic/speed differences between the metals and achieve sequential flotation and take full advantage of the hydrophobic nature of each mineral, it is best to first lower the pH and see about recovering copper. MBS is the pH modifier of choice to dropping pH into a zone where Cu is hydrophobic while all other metals are hydrophilic provided an opportunity.
As MBS is added and pH drops from Natural pH 9, other minerals are depressed is this order:
- Iron (pyrite)
- Zinc
- Lead
- Copper (Chalcopyrite and others) As Lime is added and rises pH towards 12, depressed again is Iron (pyrite), followed by Zinc.
** Activated Zinc, in the Cu and/or Pb Circuit, will float like Copper.