Table of Contents
Low recoveries of cassiterite are obtained by gravity concentration of lode deposits, particularly if the cassiterite calls for fine grinding of the ore. And it is precisely this finely ground ore that is the cause of the low recoveries.
The possibility therefore of upgrading the ore by flotation has been of great interest to the tin industry because flotation, in general, produces high recoveries from other finely-ground metal ores. Many attempts have been made to float cassiterite directly. This paper is a summary of our work on sulphidization of cassiterite, iron oxides, the dissociation of pyrite and their subsequent flotation and separation, in artificial mixtures and natural ores.
Investigation Plan
While there were many steps in our investigation plan, the major phases of our investigation were:
- Microscopic analysis of the products of cassiterite sulphidization, with the variables: (a) particle size, (b) time for sulphidization (c) temperature during sulphidization;
- Sulphidization Flotation tests using high-grade cassiterite; testing the following variables: a) Particle Size; b) Temperature of Sulphidization; c) Time for Sulfidization.
- Sulphidization – Magnetic Separation-Flotation of: a) high grade hematite ore and b) magnetite ore.
- Thermal dissociation of pyrite, testing the following variables; a) Size fraction; b) Time of dissociation; c) Temperature.
- Flotation and magnetic behavior of each of the dissociated pyrites made at: a) low temperature (580°C – 600°C) and b) high temperature (715°C – 750°C).
- Separation of cassiterite from a low-grade gravity concentrate derived from a natural ore.
Apparatus and Experimental Procedure
In order to carry out the experimental work, equipment and procedures were developed for sulphidization of cassiterite and iron oxides and for dissociation of pyrite. In addition, procedures were standardized for the subsequent magnetic and flotation separations of the products of sulphidization.
We used two different systems with hydrogen sulphide to sulphidize ores, high grade concentrates of cassiterite and iron oxides either individually or in combination with Ottawa sand as artificial mixtures. A description of the two systems follows.
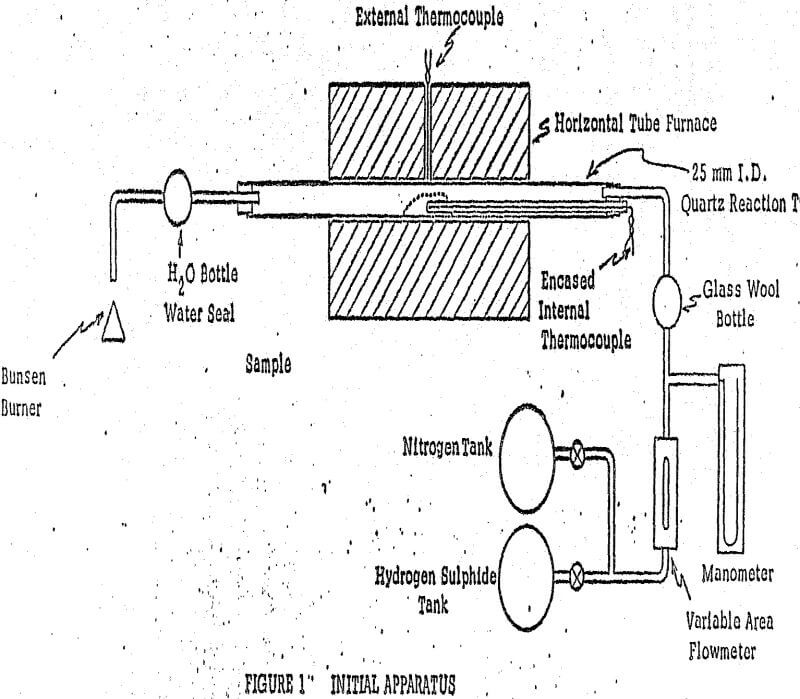
For initial exploratory tests, we used a horizontal furnace. The temperature was controlled by means of a Powerstat voltage regulator. The reactions occurred in the 25mm quartz tube within the furnace.
The ground sample was placed inside the tube. The samples were heated to the required temperature in a flow of nitrogen, allowing 15 minutes for temperature stabilization prior to the hydrogen sulphide flow. Gaseous hydrogen sulphide and nitrogen were supplied from standard gas cylinders.
To prevent compaction during sulphidization, and to obtain thorough contact of gases and solids, a fluid-bed unit was built and tried. Another factor in favor of developing a fluid- bed unit was to be able to sulphidize relatively large samples in order to have sufficient feed to test various methods of concentration and separation.
The mode of operation in a typical hydrogen sulphide sulphidization was as follows: A weighed sample was charged into the reaction tube; the cooler was placed on top of the tube and a flow of nitrogen (3 liters/min) was maintained during the heating period, in which the bed was visually well fluidized. When the control reached the temperature setting, 30 minutes of stabilization was allowed for prior to the start of the hydrogen sulphide flow.
The same operating procedures was followed with hematite, magnetite, and with ores during and after their hydrogen sulphide sulphidization.
Procedure for Thermal Decomposition of Pyrite and Sulphidization of Cassiterite by Pyrite
When only pyrite was treated for its thermal dissociation, the flow of nitrogen was maintained at 6 liters/min. This rate was high enough to carry the resultant sulphur vapor outside the reaction tube, thus preventing any build-up of sulphur pressure which might stop the reaction.
Fluidization was difficult. Although this large flow of nitrogen was used only in a few of the tests, only a small part of the bed was observed to be completely fluidized. In most of the funs the bed was static but with a very porous and sponge-like texture.
For these types of experiments we started to count the reaction time as soon as the temperature inside the tube reached the desired one. After completion of the reaction period, the power was turned off, the furnace opened and allowed to air-cool. Fans helped to circulate the air. The sampling, if necessary, and unloading of the tube, followed the same procedure listed previously.
Generally, the following reagents were used in flotation testing:
Collectors:
(a) Potassium amyl xanthate, Z-6, technical grade, from the Dow Chemical Company, used in a 0.5% freshly prepared aqueous solution; (b) Aerofloat 211, technical grade, from American Cyanamid Company, in 0.1% aqueous solution.
Frother:
Dowfroth 250, in 10% solution in absolute ethyl alcohol.
pH Regulators:
- Sodium hydroxide
- Calcium oxide
- Sulphuric acid
- Hydrochloric acid
These were analytical reagent grade in various dilute aqueous solutions.
Summary of Results
All samples of cassiterite products sulphidized at 580° – 600°C showed varying degrees of surface covering. Where the covering occurred it looked like glittering yellow-gold. For products from short reaction periods (15 min. increasing to 60 min.) there was apparently unreacted cassiterite (zinc blende-like color to translucent red) in these samples. The amount of unreacted cassiterite decreased as the period of reaction was increased.
In cassiterite samples sulphidized at 45 and 60 minute periods, small flakes of yellow-gold material appeared among the sized sulphidized particles. Some of the yellow-gold surface of the sulphidized particles had peeled off, revealing another phase below the yellow-gold one. This phase was a dull, sooty black color.
In general, for reaction time of 30 minutes and over, the cassiterite was rimmed by reacted products. In no case were any cassiterite particles completely sulphidized throughout the grain, even those at 325-mesh and at 3 hours reaction time. The extent of the rim around the cassiterite particles increased with an increase in reaction time. Thus, for 15 minute reaction times, some portion of the cassiterite surfaces were unreacted. Indeed, some grains were unreacted. Also, as reaction times in¬creased, the depth of the observed rim increased. But, in all cases this rim was relatively thin.
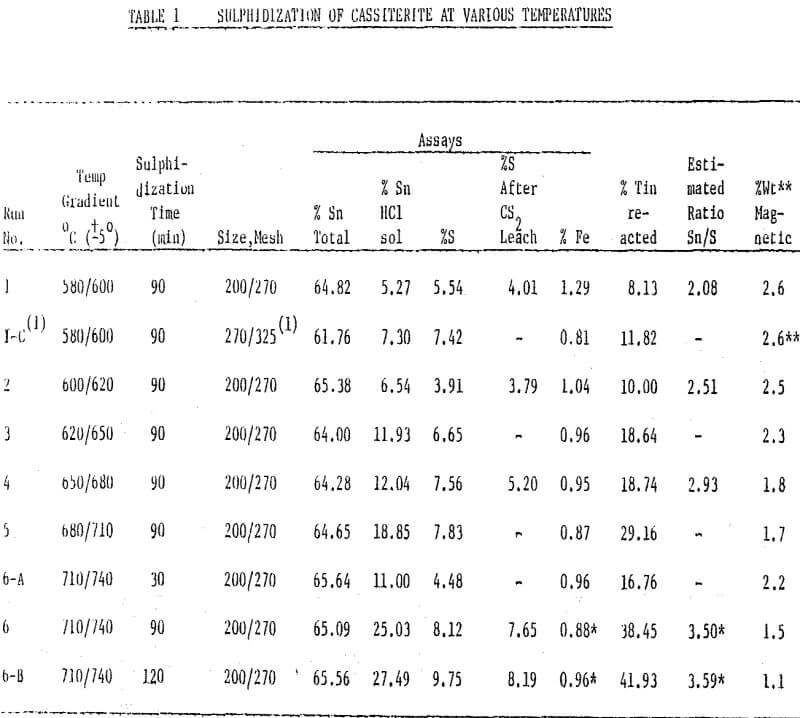
Sulphidized Products vs. Temperature
All samples or cassiterite of the size minus 200 to 270 mesh, sulphidized for 90 minutes at various temperatures between 580 and 740°C, showed surface covering. The covering appeared mostly as bulky glittering yellow-gold for the sample of sulphidization at 580° – 600°C. In this sample, some of the yellow-gold material was observed to be free. This material appeared as fine, flaky, translucent particles. In the samples sulphidized above 600°C, and up to 650° the box-work texture of the bulky yellow shell was clearly observed because a large amount of shell was peeled off, uncovering a dull black compact phase underneath. At 600° – 620°C sulphidization, there was an apparent increase in flaking of the golden shell, and an increase in. the amount of free yellow-golden fines. In the 620° – 650°C sulphidization tests, needle-like shiny fines were also present.
A plot or cumulative sulphidiztion time (min) ; vs. percent recovery of tin in the froth was plotted. Here, there is a maximum recovery for all size fractions, after 90 minutes of sulphidization. This agrees with the observation that at about this reaction period the cassiterite surface, appears to fee completely covered with stannic sulphide.
The 90% recovery level is reached in size fractions 150/200, 200/270, and 270/325 mesh after about 28, 36 and 48 minutes of sulphidization, respectively.
Pyrite is an economically attractive source of sulphur for the direct cassiterite sulphidization process. However, the yield of sulphur from pyrite dissociation becomes significant at temperatures only above 700°C.
Flotation Tests of Sulphidized Products
Cassiterite Flotation (200/270-mesh Sulphidized at 580°C – 600°C. 90 minutes)
- Flotation is accomplished easily in acid pulps with low concentrations of collector of the xanthate type;
- Flotability is decreased in alkaline pulps;
- Sodium aerofloat yields higher tin recoveries than potassium amyl xanthate in basic solutions;
- An increase of collector yields higher recoveries, particularly in alkaline pH;
- Leaching sulphidized cassiterite in hot water is harmful to subsequent flotation;
- Soluble ions from sulphidized hematite are harmful to cassiterite flotation, especially at low collector concentrations;
- Cyanide, in a 10 mg/l concentration, does not depress sulphidized cassiterite. On the contrary, it appears to counteract the harmful effect of soluble ions from sulphidized hematite;
- The use of lime as a pH modifier does not yield a significant loss in tin flotation recovery at the conditions tested for a : possible flotation separation from iron sulphides.
Cassiterite (200 – 270 mesh at 600 – 620°C, for 90 minutes )
He present the following summary of our results:
- pH is critical with potassium amyl xanthate as the collector in the flotation of cassiterite sulphidized at 600 – 620°C;
- Maximum flotability occurs at a pH very close to 2.8;
- Minimum flotability appears to exist at about pH 5.0, particularly at low collector concentrations;
- An increase in collector concentration increases the amount of material floated, especially at low pH values;
- Sequence of additions of reagents is critical. The addition of collector and conditioning prior to pH modifier increases floatability at pH values below 3.0.
These findings concerning the cassiterite flotation products having a thin sesquisulphide coating were combined with the data on flotation of Sn2S3, i.e., flotation at low pH values. These flotation conditions are: (a) flotation of iron sulphides at low xanthate concentrations at a pH value of about 5.0, and (b) possible flotation of sulphidized cassiterite at a pH value of about 2.8 at high xanthate concentrations.
To test the applicability of conclusions based on data derived from testing relatively pure materials, we tested the low grade gravity concentrate from Catavi. In these tests the flowsheet was as follows:
- The sulphidized ore was subjected to dry magnetic concentration to produce an iron concentrate;
- The magnetic tailings were floated with aerofloat at an alkaline pH;
- The tailings from step (2) were floated at an acid pH of about 5 to produce a mixed FeS2 and Sn2S3 concentrate;
- The tailings of step (3) were floated in a highly acid circuit (pH 2.8) to produce a predominantly SnS concentrate.