Table of Contents
Bastnasite rare earths are now being utilized to a limited extent as a source of rare-earth materials used in the steel industry; but advances in the technology of separating the rare-earth elements from each other will make the individual elements available in adequate supply for engineering evaluation and ultimate utilization.
The Federal Bureau of Mines is conducting an active program of research on rare earths, including analytical procedures, ore-concentrating techniques, and extraction from ores and concentrates, as well as ion-exchange and other methods of separating rare-earth elements. The program also includes studies on production of the metals by electrolytic reduction, physical metallurgy of the metals and their alloys, and determination of basic scientific data on the rare-earth metals and their compounds.
The purpose of the work described in this report was to ascertain the influence of eluant flow rate and resin crosslinkage, resin-bed shape, and the effect of separating ions on the separating phenomenon. EDTA acid was used as the eluant for these studies, and copper-cycle resin was utilized in the rare- earth-free portion of the columns after Spedding and coworkers.
Separation of the rare-earth elements, including yttrium, has long been investigated intensively. The chemical properties of the rare-earth elements and yttrium are so similar that separation by classical fractional precipitation schemes is at best an expensive and tedious process. The first practical process utilizing ion-exchange techniques for separating the rare-earth elements was developed independently by workers at the Institute of Atomic Research, Iowa State College, Ames, Iowa, and the Atomic Energy Commission (AEC) laboratories at Oak Ridge, Tenn., during World War II. Since several reviews of ion-exchange literature have been published recently, a comprehensive summary of the literature will not be repeated; however, certain pertinent works should be mentioned. F. H. Spedding and his coworkers have published a number of papers on ion-exchange separation of the rare-earth elements, using citric acid, ethylene-diaminetetraacetic acid (EDTA), and hydroxyethlenediaminetriacetic acid (HEDTA) as eluants. E. C. Frelling, D. H. Harris, S. W. Mayer, E. R. Thompkins, L. Wish and others at the U. S. Naval Radiological Defense Laboratory, San Francisco, Calif., and G. E. Boyd, B. H. Ketelle, and L. S. Myers, Jr. at the AEC laboratories have issued papers on ion-exchange theory and the use of various eluants. R. C. Vickery published a paper comparing several eluants and giving results of adding elements other than rare earths to column feed stock.
Experimental Procedure
The columns used for these experiments consisted of various sizes of glass tubing fitted with rubber stoppers and a porous material, such as Dynel, Teflon cloth, or fritted glass, to support the resin bed. Figure 1 shows a typical ion-exchange column arrangement. Columns were filled to the desired height with nuclear sulfonic acid-type cation exchange resins in the full mesh size, as received from the manufacturer. The resin fines were removed by backwashing at a rate sufficient to expand the beds 100 percent. Backwashing also served to classify the resin and insure a uniform particle-size distribution across the bed to minimize channeling.
Fresh resin beds were conditioned by elution with a 25-percent excess of a 5-percent solution of hydrochloric acid, followed by a similar solution of ammonium hydroxide: any traces of metal impurities in the resin were removed
by elution with a solution of the ammonium salt of the eluant. The beds were washed with deionized water to remove the excess ammonium-salt solution. A similar wash after each run removed excess eluant from the interstices and pores of the resin.
The rare-earth charge solution consisted of rare-earth elements leached from bastnasite concentrates assaying 55-percent rare-earth elements. Virtually all the cerium and half the lanthanum were removed by conventional chemical means; the remaining rare-earth elements were precipitated as oxalates. The oxalates were converted to oxides by ignition at 1,750° F. The mixture of rare-earth oxides was weighed, dissolved in hydrochloric acid, evaporated to near dryness, and redissolved in deionized water. A few drops of hydrochloric acid completed solution of the chlorides. The desired stoichiometric amount of the chloride solution was passed through and adsorbed on the first resin bed of each series of beds to be concatenated for an experiment. The remaining resin (free-resin) beds in the series were either left in the ammonia cycle or placed in the copper cycle by passing cupric sulfate solution through the resin until influent and effluent concentrations were identical. The rare-earth chloride and cupric sulfate solution flow rates ranged from 2 to 5 ml./min./sq.cm.
The eluting solution consisted of a 0.015 M solution of EDTA in aqueous ammoniacal solution at ambient temperature. A polyethylene displacement pump similar to an acid egg was used to force eluant through the resin beds. Flow was top to bottom, through the rare-earth-cycle resin bed and successively through the copper-cycle resin bed. A band of copper-pregnant solution (copper being in the form of copper-EDTA chelate) traveled down and out of the final column, followed by a band of chelated heavy rare-earth elements; then bands of chelated neodymium, praseodymium, cerium, and lanthanum were formed in the order given. By continued elution the effluent was collected as a series of equal-size fractions or samples, and the pH of each sample was measured.
The rare-earth elements in the effluent samples were precipitated with 5-percent excess oxalic acid, burned to the oxide at 1,750° F., weighed, and analyzed by X-ray emission spectroscopy.
Results and Discussion
The results given below include the effects of eluant flow rate, resin crosslinkage, resin-bed shape, and addition of separating ions on separation of bastnasite rare earths.
Data presented graphically in figures 2 through 7 were plotted as the amount (as percent of total rare-earth elements) of each rare-earth element present in a sample versus the accumulated volume of each sample, that is, sample analysis versus accumulated sample volume. The pH and total rare-earth concentration of each sample are plotted versus the accumulated sample volume in the same graphs. Rare-earth feed-stock analyses are summarized in table 1.

Resin Crosslinkage and Eluant Flow Rate
In nuclear sulfonic polystyrene resins porosity within the polymer network depends on the amount of crosslinkage in the polymer, the crosslinkage being DVB copolymerized with the styrene and known as “DVB crosslinkage.” The manufacturer defines a resin containing 12 percent DVB as “low porosity,” a resin containing 8 percent DVB as “medium porosity,” and resins containing 2 and 4 percent DVB as “high porosity.” In Dowex 50×12, the “12” signifies 12 percent DVB, hence the term means Dowex 50 resin with crosslinkage corresponding to 12 percent DVB.
Because chelated rare-earth elements must enter the resin matrix to exchange with other rare-earth elements adsorbed on the resin, the rate of exchange would, in part, depend on the diffusional characteristics of the resin, these characteristics, in turn, being a function of the resin crosslinkage (or porosity). Data concerning resin crosslinkage and eluant flow rate, as they apply to the bastnasite leach solution being studied, were obtained in a series of nine runs on the rare-earth mixture given in table 1.
For the data in figure 2, six 1.7-cm.-I.D. columns were filled to a height of 104 cm. with 100- to 200—mesh Dowex 50×12 resin and arranged in pairs, each pair being designated for one of three separate runs. For each run the first and second columns were saturated with rare-earth elements and divalent copper, respectively, and then connected in series. The rare-earth elements were eluted from the first column of each pair through the corresponding copper-cycle columns at flow rates of 2, 5, and 10 ml./min./sq.cm., with 0.015 molar EDTA at a pH of 8.0. Duplicate tests were made using 100- to 200-mesh Dowex 50×8 and x 4 resins to obtain data shown in figures 3 and 4. The 1.7- by 104-cm. columns of Dowex 50×12, x 8, and x 4 resin beds adsorbed 30, 27, and 19.3 grams of rare-earth elements, respectively.
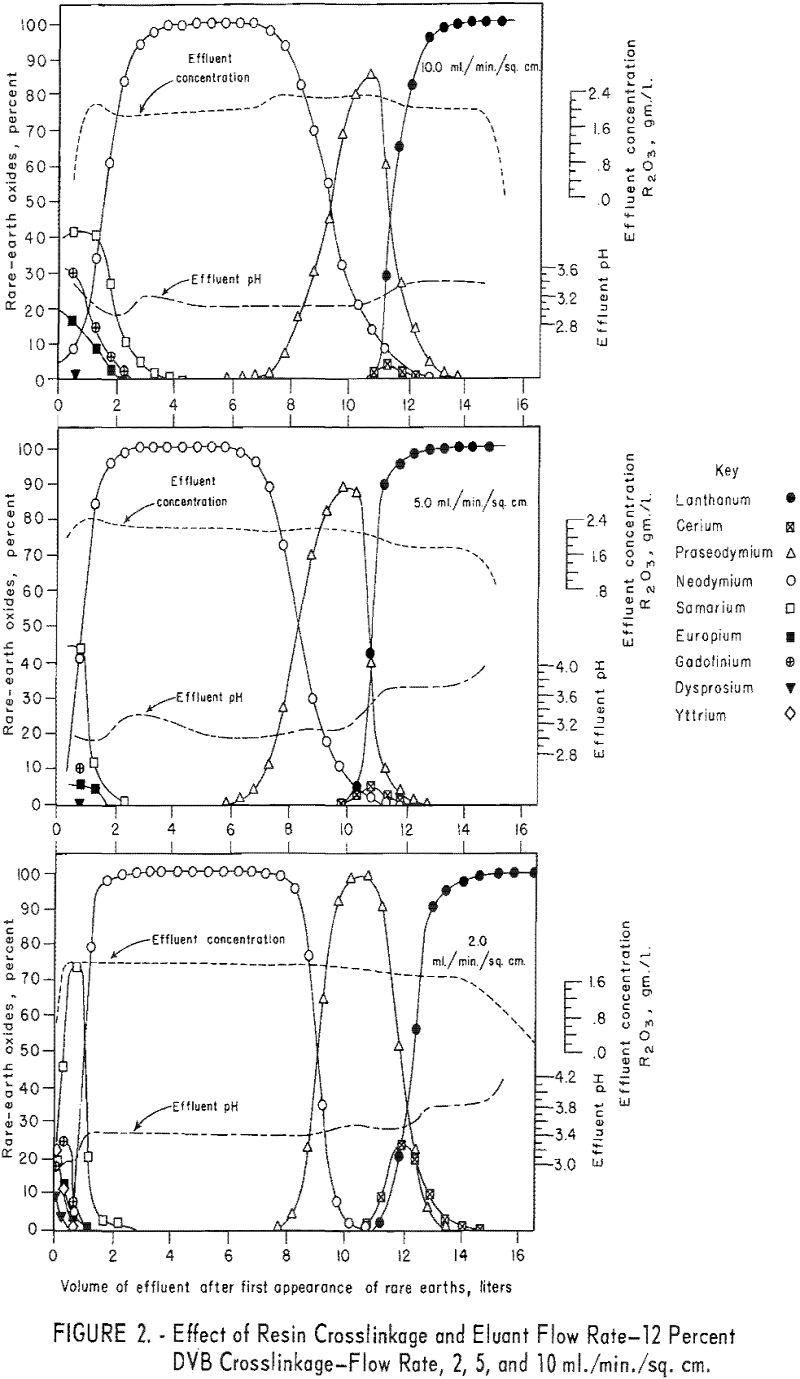
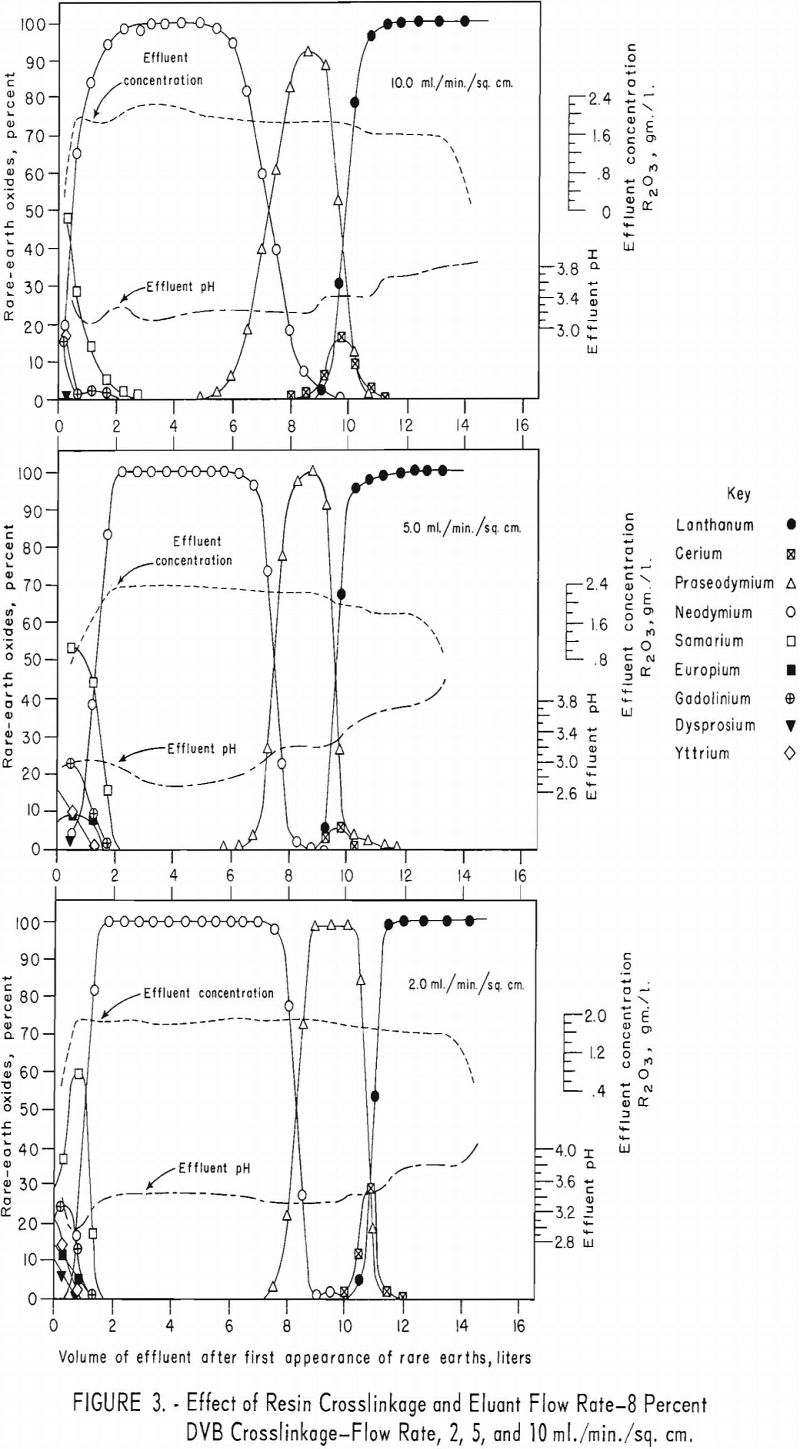
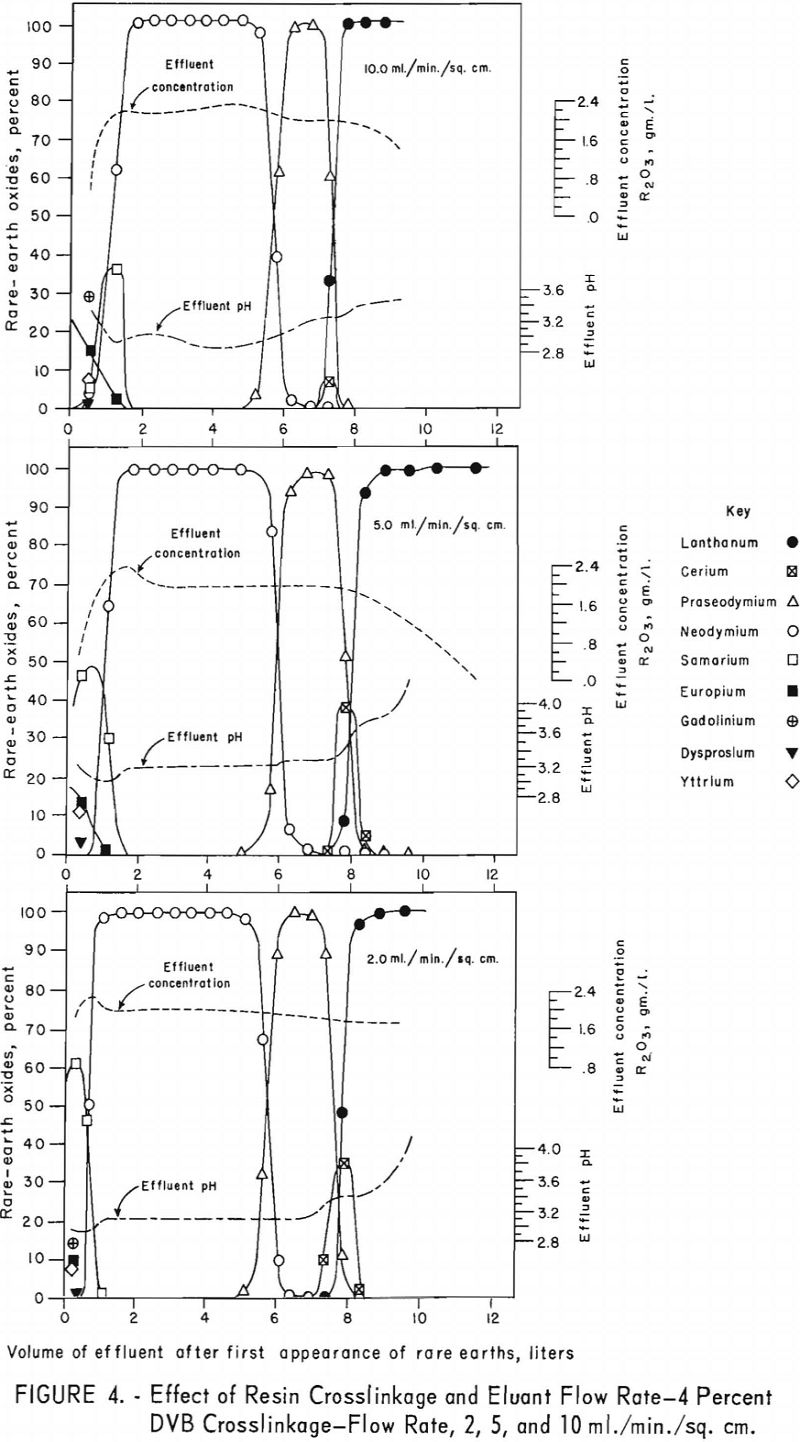
The data in figures 2 through 4 show that, with 12-percent DVB crosslinkage resins, a continuous decline in degree of separation occurs on increasing eluant flow rate from 2 to 5 and up to 10 ml./min./sq.cm. The 8-percent DVB resins resulted in separation that was relatively constant between 2 and 5 ml./min./sq.cm. and then declined between 5 and 10 ml./min./sq.cm. The 4-percent DVB resin gave nearly constant separation over the entire range of flow rates tested.
The nearly constant degree of separation obtained with the 12-, 8-, and 4-percent DVB crosslinked resins at low flow rates and the variation in degree of separation obtained with these three resins at higher flow rates is attributed to the differences in porosity of the 12-, 8-, and 4-percent DVB crosslinked resins. Low porosity in the 8- and 12-percent DVB crosslinked resins inhibits diffusion of rare-earth-EDTA chelates in and out of the resin matrix; hence, less separation is obtained with low porosity resins at high flow rates.
Resin-Bed Shape
The effect of resin-bed shape was determined with 225 ml. (wet settled volume) of both copper-cycle and ammonia-cycle resins in columns of various diameters. In a typical set of runs (fig. 5), a gradual increase in
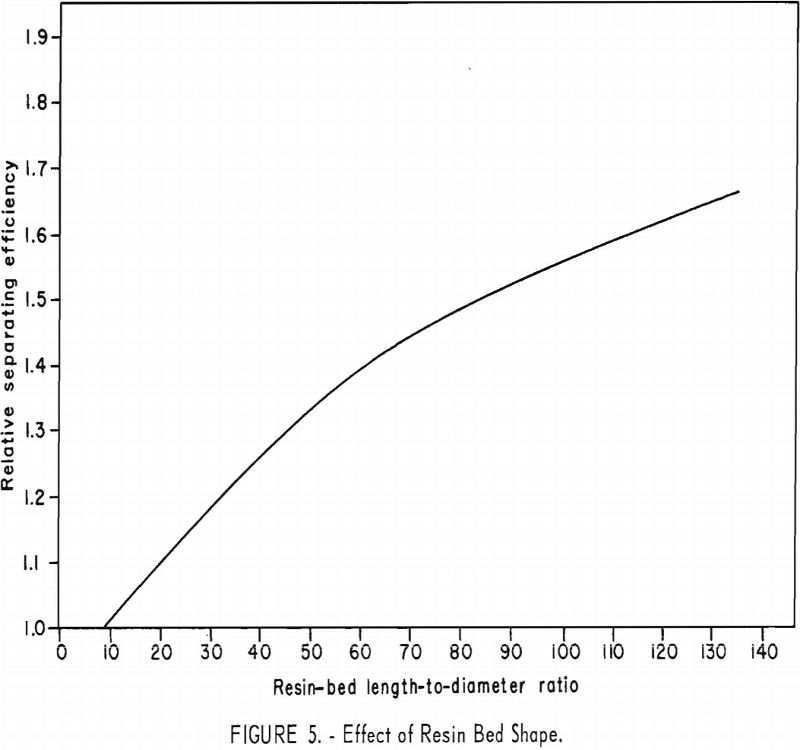
separation efficiency was obtained with increasing length-to-diameter ratios of resin bed. The rate of improvement in efficiency declines slowly as the length-to-diameter ratio increases from 9 to 135. Under these conditions the separation efficiency would be expected to continue to increase with increasing length-to-diameter ratio until wall effects become important.
The influence of resin-bed shape is easily understood when it is recognized that the height of an overlap region or band (the portion of resin containing the unseparated fraction between two adjacent rare-earth elements) is not a function of the column diameter. For example, when other conditions are the same, the height of an overlap region remains nearly the same, regardless of column diameter. This means that, for a given amount of charge to a series of ion-exchange columns, the percentage of each element recovered pure will decrease with increasing diameter of columns used, because more of each element is in an overlap region with adjacent elements.
Separating Ions
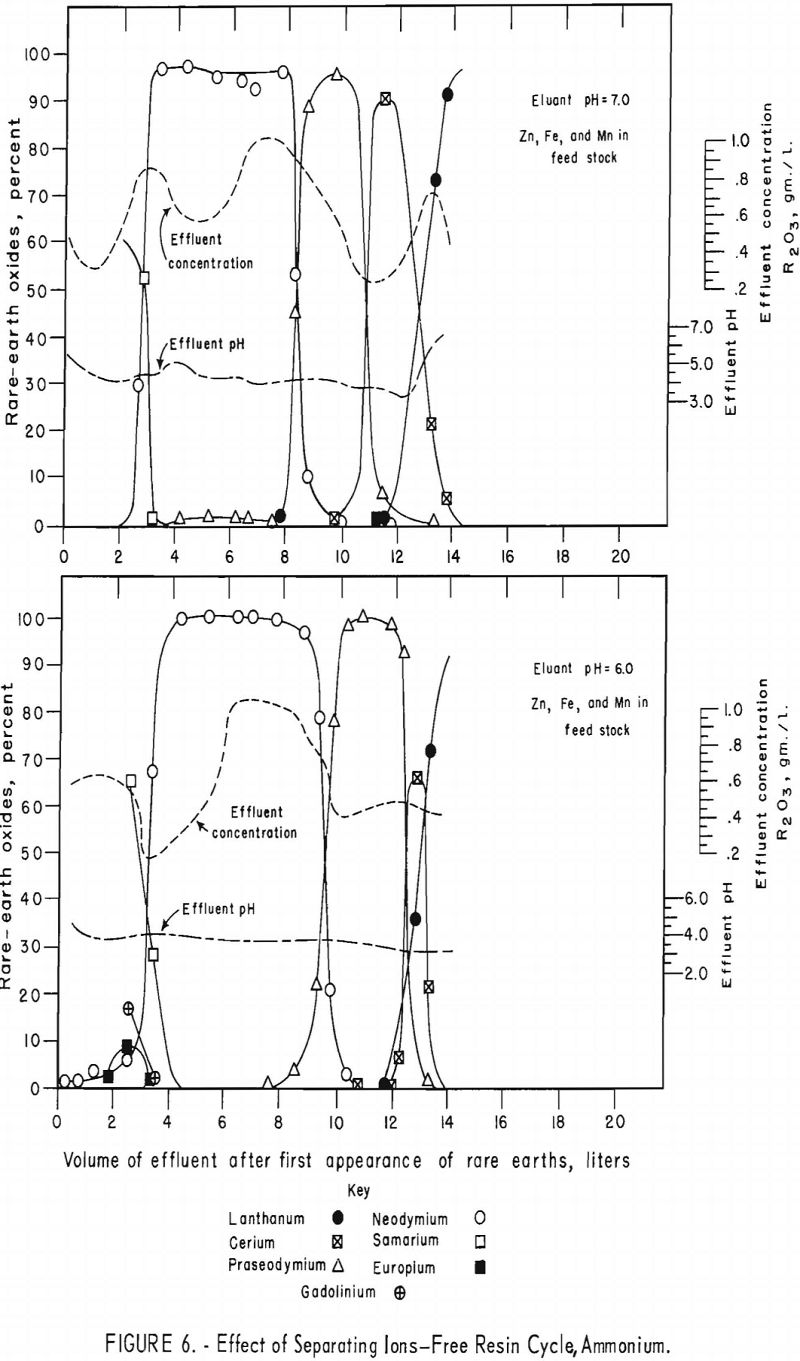
Data concerning the effects of the addition of divalent zinc, cobalt, iron, and manganese chlorides to rare-earth feedstock solution were obtained in a series of four runs.
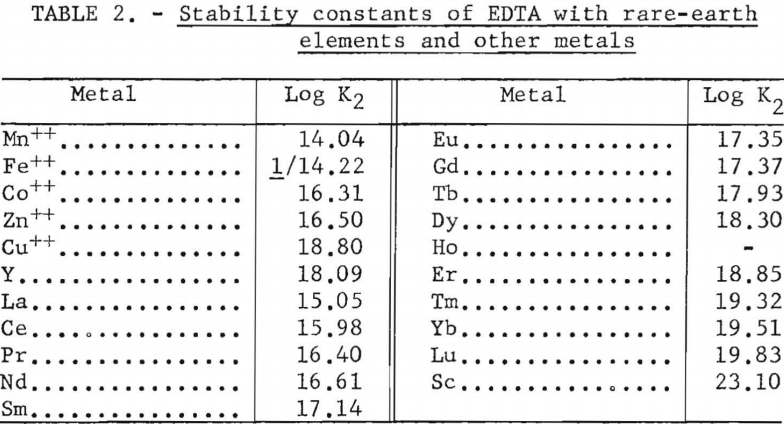
For the data in figure 6, a mixture of 2.25 grams (oxide basis) each of zinc, iron, and manganese and 7.5 grams of rare-earth feed stock was adsorbed on a 1.5-cm.-diameter by 184-cm.-high column of 20- to 50-mesh Dowex 50×4 resin in the ammonia cycle. The zinc, iron, and manganese were eluted down the column with 0.015 molar EDTA at a pH of 7.0 in one run and 6.0 in another. The flow rate was held constant at 2 ml./min./sq.cm. The zinc was eluted along with the samarium or heavier rare earths in both runs. The iron was eluted with the neodymium at a pH of 7.0 but shifted toward the samarium at a pH of 6.0. The manganese was eluted with praseodymium and cerium at a pH of 7.0 but shifted toward the heavy rare earths and was eluted, for the most part, with the praseodymium at a pH of 6.0. This is shown by the low rare-earth-concentration regions in figure 6. The shift of iron and manganese toward the heavier rare-earth elements at lower pH values probably is due to weakening of the chelate at lower pH values, thereby increasing the influence of the resin selectivity (metal+³>metal+²) relative to the stabilities of the metal ions with EDTA (table 2); that is, the resin would have an increased tendency to retain the trivalent rare-earth elements longer and pass on the divalent iron and manganese. Vickery reported that, with an EDTA concentration of 0.0052 molar and ammonia-cycle free resin, iron eluted between samarium and neodymium; however, in his work the manganese appeared between the neodymium and praseodymium fractions.
For the first of the next two runs (fig. 7, upper), a mixture of 40 grams of rare-earth elements and 20 grams each of divalent zinc, cobalt, and manganese was adsorbed on two 3.2-cm.-diameter by 78-cm.-high columns of 16- to 50-mesh Amberlite IRC-120 resin. The rare-earth elements and the zinc, cobalt, and manganese mixture were eluted through one 3.2-cm.-diameter by 127-cm.-high column of 16- to 50-mesh Amberlite IRC-120 resin in the copper cycle and one 2.2-cm.-diameter by 127-cm.-high column of 100- to 200-mesh Dowex 50×4
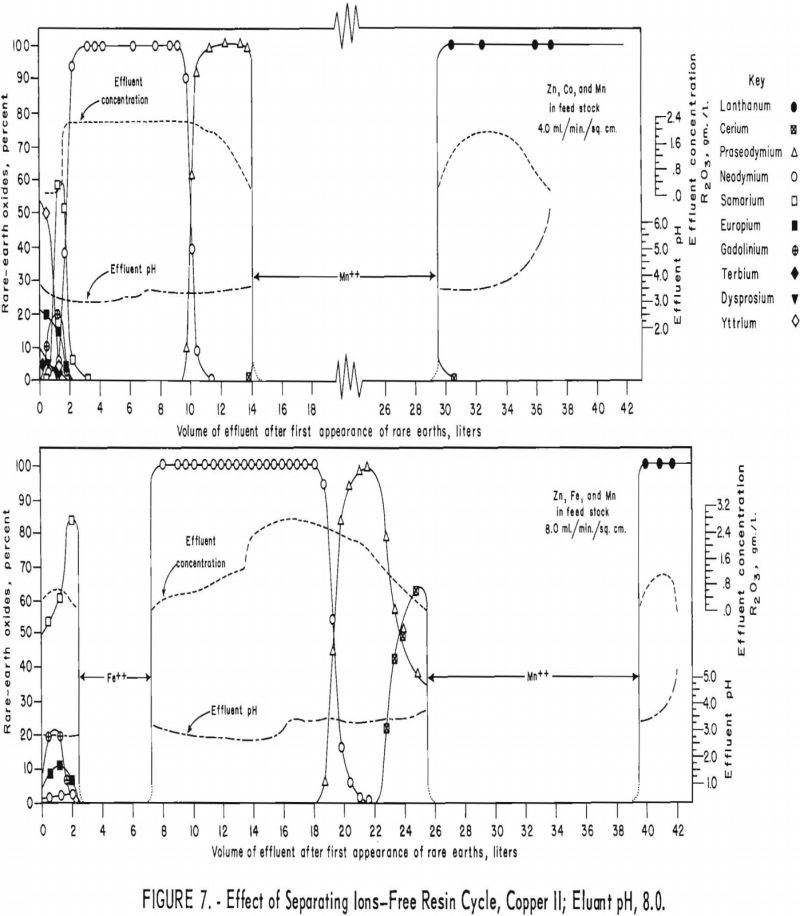
resin in the copper cycle with 0.015 molar EDTA at a pH of 8.0 and flow rate of 4.0 ml./min./sq.cm. The second run (fig. 7, lower) was similar to the first, except that iron was substituted for cobalt and the eluant flow rate was 8.0 ml./min./sq.cm. Under the conditions used for these runs, zinc and cobalt were eluted with the samarium and heavier rare-earth elements. The iron effected complete partition of samarium and neodymium but trailed into the neodymium considerably. Manganese effected partition of lanthanum and praseodymium in the first run (fig. 7, upper) and of lanthanum and cerium in the second run (fig. 7, lower), but it also spread itself into adjacent rare-earth fractions considerably.
Conclusions
DVB crosslinkage of the resin, by virtue of its relation to resin porosity, influences separation efficiency by affecting the rate of diffusion of the rare-earth chelates to and from the exchange sites. Twelve percent DVB crosslinkage (low porosity) impairs separation at flow rates over 2 to 5 ml./ min./sq.cm., whereas 4-percent DVB crosslinkage (high porosity) permits flow rates up to 10 ml./min./sq.cm. without impairing separation.
Resin-bed shape increases separation efficiency as the length-to-diameter ratio is increased over the range from 9 to 135; however, the rate of improvement in efficiency declines as the ratio increases.
Divalent iron and manganese, added to rare-earth feed stock as separating ions, elute with and between and effectively separate samarium from neodymium and cerium from lanthanum, respectively. The results of adding zinc and cobalt were inconclusive. These elements were eluted in a mixture with samarium and heavier rare-earth elements; however, the heavier rare-earth elements were present in quantities too small to be separated under the conditions used.
