Table of Contents
- USBM Process
- Equilibrium Tests
- Column Tests
- Results of Equilibrium Batch Tests
- Results of Column Tests
- Mechanisms of Ree Elution from Sulfonic and Iminodiacetic Resins
- Effects of Variables
- Flow Rate
- pH
- Recycle of Mixed REE
- Separation of REE and EDTA
- HCl Precipitation of EDTA
- REE-Oxalate Precipitation and Roasting
Potential increases in overall demand and purity requirements of rare-earth elements (REE) prompted the U.S. Bureau of Mines (USBM) to investigate new technology for separating these elements.
REE, as defined by the USBM, include a group of 17 elements composed of Sc, Y, and the lanthanides. Although exact numbers are not available because of proprietary information, domestic demand for REE in 1993 was estimated to be higher than in 1992. Current market treads are toward high-purity products and away from mixed concentrates; therefore, future supply problems may be encountered if a large demand is developed for one or more of the less abundant REE.
The United States is self-sufficient in light REE resources due to the large REE deposit and processing facility at Mountain Pass, CA, operated by Molycorp, Inc. Separation of the mixed REE, however, is a complicated solvent extraction process involving many mixer-settler units. The Molycorp operation separates Ce from flotation concentrate in a hydrochloric acid (HCl) leach with Ce left in the solid leach residue. An initial solvent extraction on the leach liquor separates La, Nd, and Pr from the heavier REE, which are reprocessed to recover Eu. Another REE-processing company, Rhone-Poulenc, operates a separation plant in Texas and another in France, where 1,000 mixer-settler units are reportedly used in the process system.
Although solvent extraction successfully supplies demands for high tonnages of light REE, hundreds more mixer-settler stages and several weeks of processing time to reach equilibrium would be required before individual heavy REE could be produced. This complex processing scheme of changing mixer-settler cells for each REE makes solvent extraction ill suited for separating heavy REE at increased future demand or even at the current level of demand. Solvent extraction generates many harmful waste chemicals that must be destroyed. A significant amount of research has been performed in the past to simplify separation of these elements through ion-exchange systems. The ion-exchange system proposed in this report generates even fewer waste chemicals than the current ion- exchange system by extensive recycling.
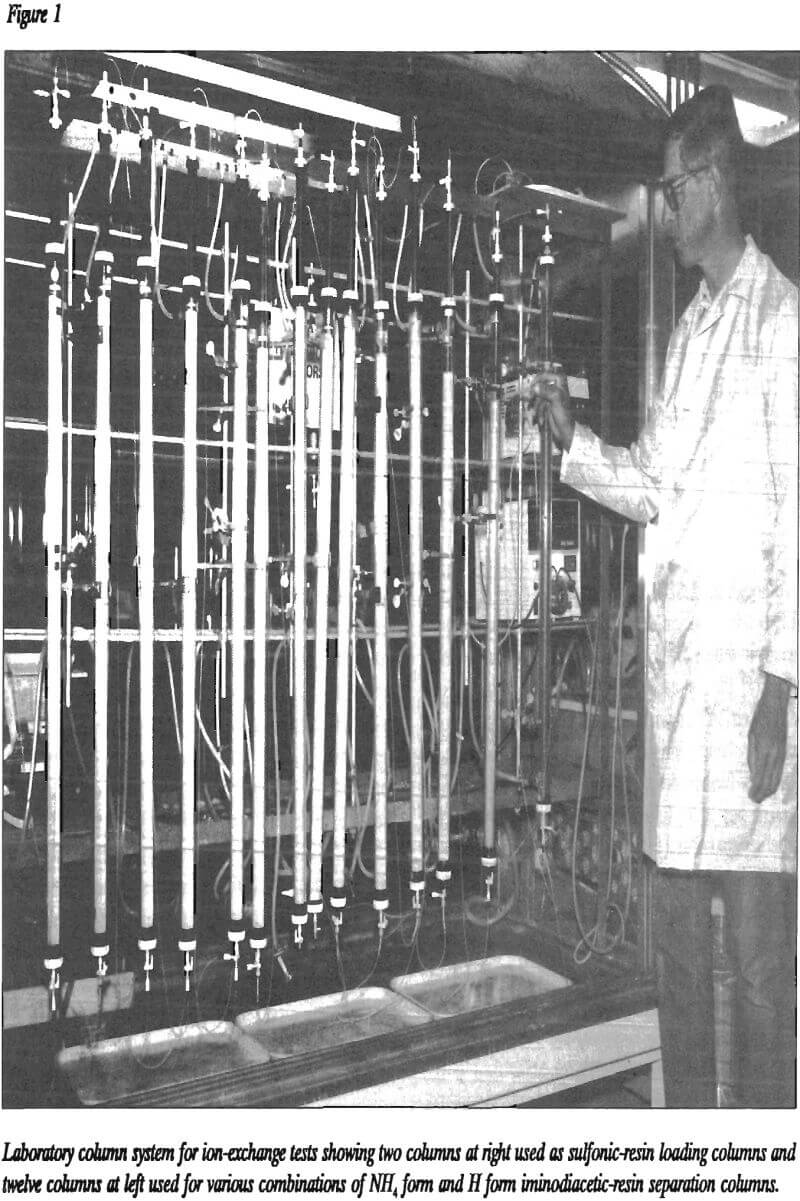
Ames Laboratory and Oak Ridge Laboratory separated rare earths by ion exchange in the late 1940’s using a sulfonic resin. Introduction of a chelating agent to the aqueous eluent led to significant improvements in the separation. In this separation, an equilibrium existed among the chelate, the different REE, and the strong cation resin, which made the separation possible. Use of ethylenediaminetetraacetic acid (EDTA) was a major improvement over the use of other chelating agents such as citric acid. Table 1 lists the published equilibria for the REE pairs between EDTA and sulfonic resin. Since a separation factor of 1.0 indicates no separation, the separation of Sm and Eu, Eu and Gd, Tm and Yb, and Yb and Lu are the most difficult separations. Amino acids similar to EDTA were found to separate some REE but could not separate as many as EDTA.
A separation mechanism referred to as displacement chromatography is initiated by a retaining ion loaded onto a strong cation exchange resin (i.e., sulfonic resin) in a separation column. A solution of mixed REE (i.e., mixed-rare-earth band) is saturated on the sulfonic resin in another column called the loading column. An eluent (i.e., EDTA) with a greater chemical attraction for the retaining ion than any REE in the mix band is pumped through the loading column and then through the separation column. In this process, the EDTA has a greater affinity for heavier REE than for lighter REE and the lighter REE replace heavier REE on the resin, and the mixed REE are aligned on the resin, heavy to light. The retaining ion prevents the REE from leaving the resin unless it exchanges with other cations many times. Several REE have distinctive colors that were observed on the resin by the early researchers, hence, the process was named chromatic displacement. Several retaining ions have been used, including Cu, Zn, and H; but they all have disadvantages. The Cu EDTA complex is very stable and Cu recovery is difficult; Zn allows the heavier REE to bleed through without exchanging on the resin; and H requires high temperatures, typically between 90 and 120 °C, and, therefore, high pressures, to prevent precipitation of H4EDTA within the resin matrix. Ion-exchange processes for separation of REE are still time-consuming and slow, lasting up to 5 to 6 months between the charging of mixed REE to the system and collection of the final separated elements.
Attempts have been made to elute chelating renins with various inorganic aqueous solutions. One report stated that using chelating resins with a chelating eluent separated rare earths poorly. A 1987 patent on recovery of Sc from tungsten ore residues claimed that dilute acid (pH 1.9 to 2.1) will elute adsorbed REE from iminodiacetic resin in the H form without eluting the Sc.
Before selecting sulfonic resin (manufactured by Bio-Rad under the trade name AG50X12) and iminodiacetic resin (manufactured by Rohm & Haas under the trade name IRC-718), EDTA eluent, and the REE retaining ion system; other resins, eluents, and retaining ion systems were screened. Most systems do not separate the REE. The BioRad and Rohm & Haas resins studied were DPI, GT73, IC120, IRC-718, AG50, C467, and the polymorphic resin XAD-4 impregnated with different REE solvent extraction reagents. Chelating and ionic eluents were used.
As part of its mission to ensure that the Nation has an adequate, secure source of minerals under acceptable environmental and economic conditions, the USBM recently developed ion-exchange technology for separating heavy REE that should decrease processing time and simplify the process. This report describes the USBM ion-exchange system that was developed.
USBM Process
Although the USBM process developed for separating REE builds upon ion-exchange research previously conducted, it differs from that research in several specific ways: (1) the ion-exchange column has two sections, a loading section containing sulfonic resin and a separation section containing iminodiacetic resin; (2) the separation section is further divided into two segments, the first segment has iminodiacetic resin in the NH4 form for partial separation and high storage capacity of REE eluted from the loading column, and the second segment has H form iminodiacetic resin for final separation of the REE; (3) EDTA solution containing mixed REE is recycled to the ion-exchange columns without additional treatment; and (4) a heavy rare-earth ion is used as the retaining ion. Since this rare-earth ion is part of the mix in most REE ores, contamination is less of a problem than if the retaining ion were not part of the mix. This section describes equipment and procedures used in conducting batch equilibrium tests and semicontinuous column tests. In these semicontinuous tests, EDTA eluent was pumped through both column sections in series: first through the loading section and then through the separation section. EDTA eluent was prepared by dissolving solid H4EDTA in water at the desired concentration and adjusting the pH to desired levels with ammonium hydroxide (NH4OH).
Equilibrium Tests
The AG50X12 resin used in this study was sized 50 to 100 mesh. The IRC-718 resin used in this study was sized 17 to 35 mesh. Affinities of these two resins for pairs of REE adjacent to each other on the periodic table were evaluated in batch tests with a separatory funnel and mechanical wrist shaker. Each resin was saturated with the rare-earth ion pairs from chloride or nitrate solutions containing equimolar concentrations of the two adjacent elements. Twelve pairs of adjacent rare earths were saturated on separate 100-g lots of H form iminodiacetic resin and six pairs of adjacent rare earths were saturated on separate 10-g lots of sulfonic resin. Sulfonic resin loads approximately 10 times as much REE as does iminodiacetic resin; therefore, the different lot sizes for the two resins were selected to keep equivalent total loadings of REE. In each test saturated resin was rinsed with deionized water, drained of excess water, and then combined in a separatory funnel with 100 mL of 4 g/L EDTA that had been adjusted to pH 8.0 with NH4OH. This mixture was agitated on a mechanical wrist shaker for 40 min, after which the EDTA solution was drained, the resin was rinsed with deionized water, and residual adsorbed REE were stripped with HCL. REE solutions were analyzed by sequential inductively coupled plasma techniques with an accuracy of ± 10%.
Column Tests
Column experiments were conducted with Amicon 2.2-cm-inside-diameter ion-exchange columns equipped with water jackets, resin-support nets, column-space adjusters, low-volume three-way valves, and low-volume tubing. Figure 1 is a photograph of the complete column system showing the loading section, consisting of two columns at the far right, and the separation section, consisting of various combinations of the remaining twelve columns at the left.
For most tests in this research study, resins in the loading section were converted to NH4 form resin and loaded with REE from chloride solutions. These chloride
solutions contained between 5 and 6 g/L of the REE and were approximately equimolar in the following elements: Gd, Tb, Dy, and Ho. Sources of REE for testing included oxides of Er, Ho, Dy, Tb, Gd, Nd, and Pr from Sinochem, Inc.; and other rare-earth salts from Alfa/Johnson Matthey. Chloride solutions were prepared from these salts.
Highly cross-linked sulfonic and iminodiacetic resins were used to limit resin expansion as the ion form of the resin changed during elution. If the resin is not highly cross-linked, such resin expansion causes compression and subsequent high pressure drop at moderated eluent flow rates, making industrial use of the resin difficult. The AG50X12 resin is a sulfonated copolymer made of poly-styrene with 12% divinylbenzene added to the polystyrene to limit swelling of the resin; the resin is said to have 12% cross-linkage. The IRC-718 is also a highly cross-linked copolymer whose percent cross-linkage is proprietary. Experimental work indicated that iminodiacetic resin expands 5% and sulfonic resin expands 7% during the ion form change that occurs during elution.
Mixed rare-earth solution was pumped through the resin in the loading section until the resin was saturated, after which the columns were rinsed with deionized water.
Sulfonic resin was used in the loading section for most work discussed in this report. Retaining ion was loaded from either chloride or sulfate solutions onto resin in the separation section in a similar manner. Both sulfonic and iminodiacetic resins were tested in the separation section.
A peristaltic pump metered eluent through the loading section and then through the separation section. Except where otherwise noted, an eluent solution of 4 g/L EDTA buffered with NH4OH was pumped through the columns at 19 mL/min (superficial linear velocity of 5 cm/min). A Model 201 Gilson fraction collector collected equal volume samples, usually 500 mL. A heated water bath with circulating water pump maintained column temperatures at 60 °C. Air was removed from deionized rinse water with a Model B2 Marvic vacuum pump and from rare-earth and acid solutions by boiling so that bubbles would not form in the resin bed and disrupt the plug flow of eluent in the resin bed. Separation of REE pairs occurs in a given height of resin and to help minimize this height of resin, it is important that there is no disruption in the plug flow of the eluent that would cause back mixing of the eluent. EDTA was assayed by ion chromatography and NH4 by an ion-sensitive electrode.
Results of Equilibrium Batch Tests
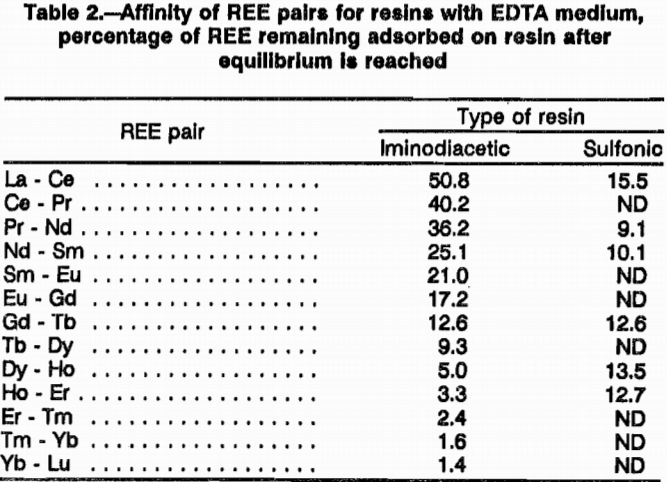
Equilibrium tests showed that iminodiacetic resin has a low affinity for heavy rare earths and a high affinity for light rare earths in an EDTA media. These tests also showed that sulfonic resin has about equal affinity for heavy and light rare earths in an EDTA media. Table 2 lists the total percentage of the rare-earth pairs that re-mained adsorbed on both resins after equilibrating with pH 8 EDTA. This difference in affinity may be responsible for the superior separation achieved with H form iminodiacetic resin. The affinity of this resin for the light REE; Nd, Pr, Ce, and La; is so great that EDTA will not quantitatively elute them from the resin. Separation factors for the REE pairs were not measured for the iminodiacetic-resin-EDTA system, but the great difference in affinity gives an indication the iminodiacetic resin in an EDTA media is sensitive to different REE.
Results of Column Tests
Semicontinuous column tests provided data for postulating elution mechanisms for the two different resins, established the effects of process variables on the two-resin system, and considered the feasibility of recycling the narrow bands of mixed-REE-EDTA solutions back through the system.
Mechanisms of Ree Elution from Sulfonic and Iminodiacetic Resins
EDTA elutes REE from iminodiacetic resin by a different mechanism than from sulfonic resin, as evidenced by the different molar ratios of REE, NH4, and H to EDTA in eluate from the two resins. This section of the report presents the different mechanisms as proposed in the current study.
Sulfonic Resin
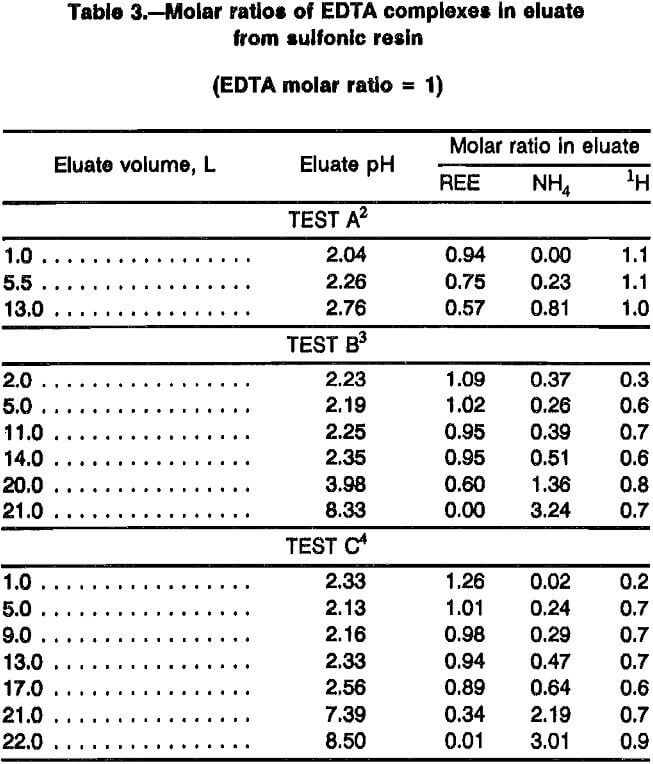
Elution of REE from sulfonic resin proceeds according to the displacement chromatography concept. Data were collected in this study to determine stoichiometry of this process. Table 3 presents molar ratios of REE, NH4, and H to EDTA in eluate from sulfonic resin for three elution tests based on chemical analysis. Eluent in test A from this table was adjusted with NH4OH to a pH of 6; eluent in the other two tests were adjusted to pH 8.4 to 8.5. Averaged assay results from eluent yielded the following EDTA:NH42:H molar ratios: pH 6 eluent, 1:2.3:1.7; and pH 8.4 eluent, 1:3.3:0.7. Test A in table 3 shows that pH 6 EDTA eluent does not carry as much REE as does pH 8.4 eluent as would be expected in displacement chromatography. EDTA buffered to pH 6 carries about 8.6 mmol/L of REE while pH 8.5 EDTA carries about 12.7 mmol/L. Tests B and C in table 3 show that after REE are eluted from the sulfonic resin with pH 8.4 and 8.5 eluent, the eluate molar ratios return to those of fresh eluent. Test A in table 3 shows some indication that this will also happen with pH 6 eluent with 14 to 15 L of eluent. A test (refer to table 7) with similar conditions shows a volume of 14 L, but this test did not assay for EDTA. These data (refer to tables 3 and 7) show that a single 80-cm-long loading column of sulfonic resin saturated with REE required between 21 and 22 L of pH 8.5 eluent to strip adsorbed REE in the laboratory system, and EDTA:REE ratios continually decrease during elution of the loading column.
Data from table 3 lead to the following approximate elution stoichiometry for early displacement of REE from sulfonic resin using pH 8.5 eluent:

As discussed above, H can be used as a retaining ion for sulfonic resin; and equation A shows no H adsorbed on the resin. Any H associated with EDTA eluent passes through sulfonic resin without adsorption changes. Adsorption of NH4 ions onto sulfonic resin leaves the resin ready for the next mixed-REE loading cycle.
Iminodiacetic Resin
The mechanism postulated for elution of REE from iminodiacetic resin has been called selective elution, and data were also collected in this study to determine the stoichiometry of this mechanism. Table 4 presents molar- concentration data from two elution tests: one with pH 6 and one with pH 8.4 eluent. Data from this table show that, initially, some EDTA-REE complex accumulates on the iminodiacetic resin, as evidenced by lower EDTA concentrations in the eluate at the start of the elution and higher concentrations at the end of the elution. Note that the molar concentration of EDTA and REE are nearly equal during the REE elution for both the pH 6 and pH 8.4 elutions. Table 5 presents molar ratios of REE, NH4, and H to EDTA in eluate from elution tests using an 80-cm sulfonic-resin loading column followed by an iminodiacetic-resin separation column with various proportions of NH4 form and H form resin.
During the first part of an elution with pH 8.4 eluent, eluate from the sulfonic-resin loading column enters the separation column with the proposed composition described by the right-hand side of reaction A. REE displacement from iminodiacetic resin during this period has a very complex mechanism. Tests A and B in table 5 show that the first eluate exiting the separation column has much lower molar ratios of NH4 and H to EDTA than did the feed to this column, both with pH 6 and pH 8.4 eluent input to the loading column. This implies that both NH4 and H are adsorbing on the iminodiacetic resin during this portion of the elution cycle. During the course of the elution, the NH4:EDTA molar ratio increases gradually to approximately 1:1 after the 21 to 22 L of eluate needed to strip the loading column has exited the separation column with pH 8.4 EDTA. The pH 6.0 EDTA requires an estimated 28 to 30 L to strip the REE from the loading column. Hydrogen:EDTA ratios decrease during this period of the elution, reaching an average level of 0.27:1.
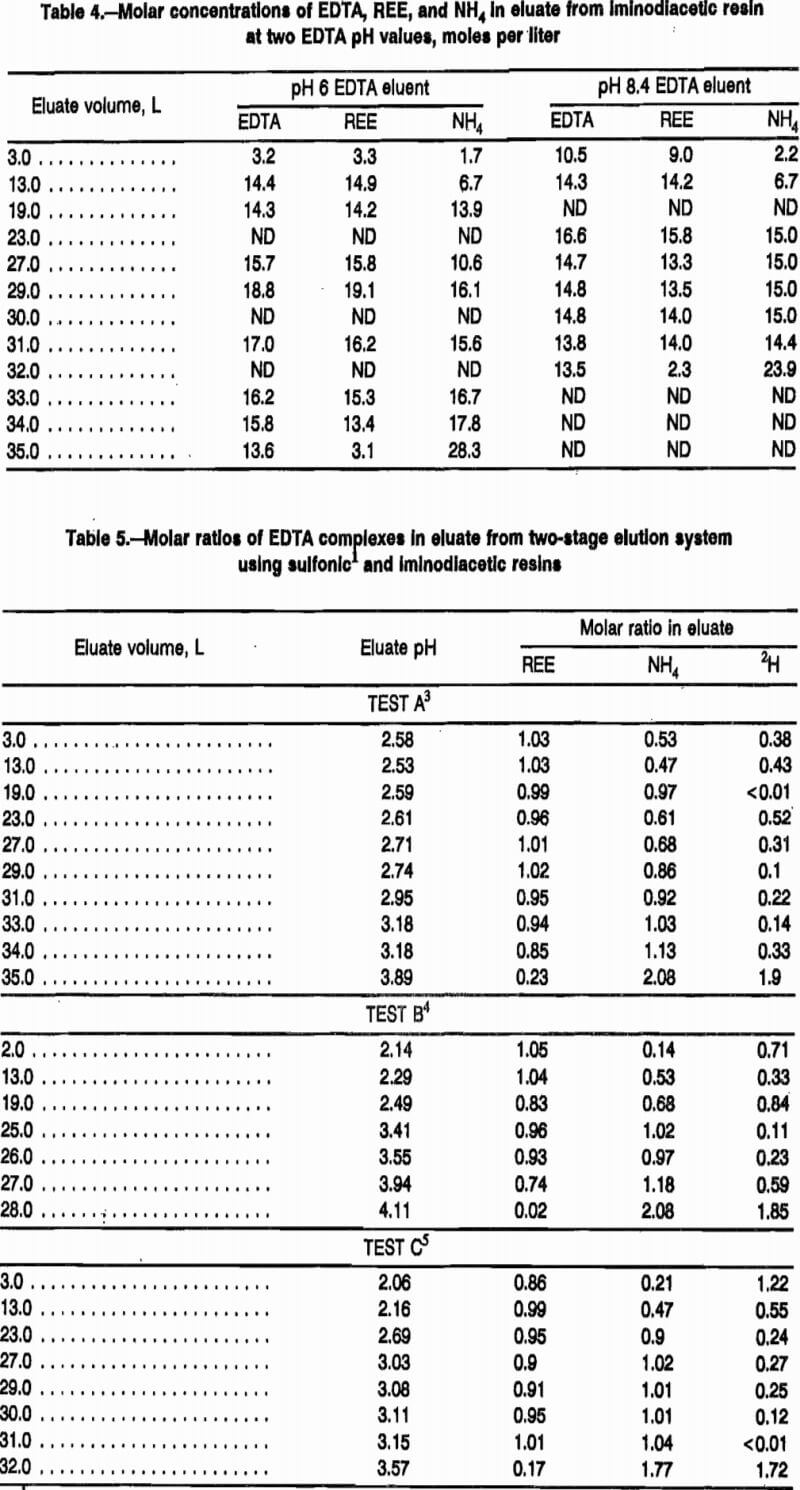
After this 21-L elution for pH 8.4 EDTA or 30 L for pH 6.0 EDTA, fresh eluent begins entering the separation column; and the eluted complex continues, as shown in table 5, with molar ratios of EDTA:REE:NH4:H having the following approximate proportions: 1:1:1:0. Each mole of EDTA carries 1 mol of NH4 until all of the REE is stripped from the iminodiacetic resin in the separation column. The proposed stoichiometry for selective elution of REE from either H or NH4 form iminodiacetic resin with pH 8.4 EDTA is represented by reaction B.
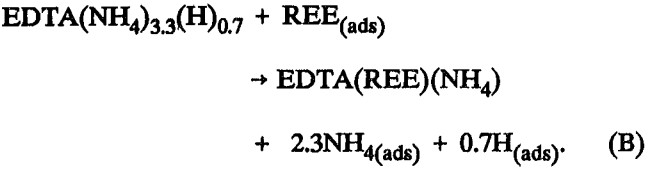
This is significantly different than the eluted species from the sulfonic resin and signifies a different mechanism of elution. The dramatic decrease of H in the eluted com-plex accounts for the higher pH levels in eluate from iminodiacetic resin.
Finally, table 5 shows that after REE were stripped from the iminodiacetic resin, the eluate did not return to the same molar ratios as those of fresh eluent as occurred with sulfonic resin. The eluate exiting iminodiacetic resin after complete elution of REE had respective EDTA:NH4:H molar ratios of 1:2:2, signifying that NH4 from the fresh eluent continued to replace H on the resin. Before all the REE is stripped from the iminodiacetic resin, EDTA eluent upstream of the remaining adsorbed REE has these same respective ratios of 1:2:2. When a REE is encountered on the resin, EDTA exchanges one NH4 and two H ions for the REE. Table 5 shows EDTA eluates with this final 1:2:2 ratio for both pH 6 and pH 8.4 eluent feed, meaning that less NH4 was adsorbed on the resin from the pH 6 eluent. This indicates that the resin is put into the NH4 form with pH 6 eluent more slowly than with pH 8.4 EDTA.
As shown by these data, the elution mechanisms for sulfonic and iminodiacetic resins are different. Displacement chromatography is the mechanism operating with sulfonic resin, and selective elution is the mechanism operating with iminodiacetic resin. Process variables were investigated for their effects on elution under these two mechanisms.
Effects of Variables
As part of the experimental work, the following process variables were investigated, some in detail, others in a cursory fashion: retaining ion, resin type, column length, eluent flow rate in the column, temperature, eluent concentration, and eluent pH.
Retaining Ion
Some early tests in this study were conducted with Cu retaining ion loaded on either sulfonic or iminodiacetic resin in the separation column. REE bled through the Cu retaining ion with little separation on iminodiacetic resin. Later tests used Yb or Er as the retaining ion and showed much better separation and very little bleed through.
Erbium and/or ytterbium have several advantages over copper as a retaining ion: (1) they are REE found in most rare-earth deposits and so they would not introduce contaminating elements into the process; (2) processes used to recover REE from the separated streams bearing single elements could be used to recover the retaining ion for recycle to the system; and (3) REE elute in the order of heaviest to lightest; therefore, they would be displaced by any lighter REE, thus minimizing bleed through of other REE. Most of the tests described in this report used Er as the retaining ion.
Resin Type
Several types of resin were screened for REE separation capabilities, and two types of resin were selected for further consideration in this study: sulfonic resin and iminodiacetic resin. Sulfonic resin is a strong cation- exchange-type resin with one exchange site on the functional SO3- group. Iminodiacetic resin is a chelating resin using two acetic acid groups as functional exchange sites, but the exchange capacity of this resin is also dependent on resin conditioning and eluent pH. Because these two resins have different loading and separation capabilities for REE, they were studied for suitability in the loading column, the separation column, or both.
Loading Column
Loading experiments showed that sulfonic resin saturated with either NH4 or H will load about 10 wt % REE. The chelating resin IRC-718 has a variable number of exchange sites on its structure that are activated when conditioned at different eluent pH. An ammonium EDTA eluent at pH 8.5 will condition iminodiacetic resin into an “NH4 form” that loads about 3 wt % REE. Iminodiacetic resin can be conditioned with a more basic eluent and its REE loading would be higher, but rare-earth oxide (REO) precipitates in the resin when the resin is rinsed with water. The IRC-718 resin downstream of where the pH 8.5 EDTA was adsorbing its NH4 and liberating H (when the REE elution occurs) is said to be in the “H form” and loads about 0.3 wt %. A more acidic conditioning of the IRC-718 resin would load less REE.
The high loading of sulfonic resin makes it preferable for the loading section of the ion-exchange column. Although separation is more selective with iminodiacetic resin, separation is not the main objective of the loading column. Here the objective is to transfer as much REE as possible to the resin at the lowest cost, an objective that is better achieved with the higher loading sulfonic resin. What separation difference exists between the two resins in the loading-column effluent is overshadowed by the fact that much less sulfonic resin is required and capital investment costs for equipment are, therefore, much less.
Separation Column
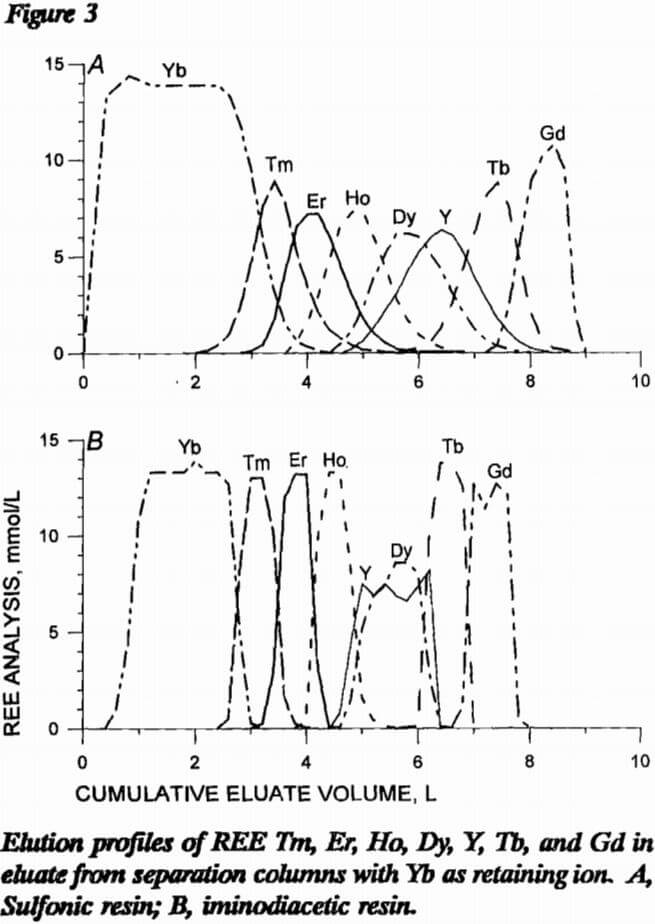
Both resin types were compared for use in the separation column using pH 8 EDTA eluent at a flow rate of 1 cm/min. Four different comparison experiments were performed. In the first two experiments, a loading column of sulfonic resin was saturated with 11 mmol each of Ho, Dy, Tb, Gd, Eu, and Sm; and sulfonic and iminodiacetic resins were tested in the separation column. With sulfonic resin in the separation column, 39 mmol Er retaining ion was loaded onto the resin; with H form iminodiacetic resin, 28 mmol Er retaining ion was loaded onto H form resin. Figure 2 presents elution profiles for these two experiments, respectively, with REE concentration in millimole per liter of eluate plotted against cumulative volume of eluate exiting the separation column. Separation with iminodiacetic resin was much sharper with smaller mixed bands of REE. The difficult-to-separate Eu was concentrated into a much smaller volume of eluent with the iminodiacetic resin. Each REE is mixed with most of the other six REE using the sulfonic-resin separation column, while only one or two REE are mixed with a given REE with the iminodiacetic-resin separation column. Data from these two resin tests are presented in table 6 as the volume of EDTA in the mixed bands between adjacent REE. Sulfonic resin produced approximately four times the mixed-REE eluate volume than did iminodiacetic resin.
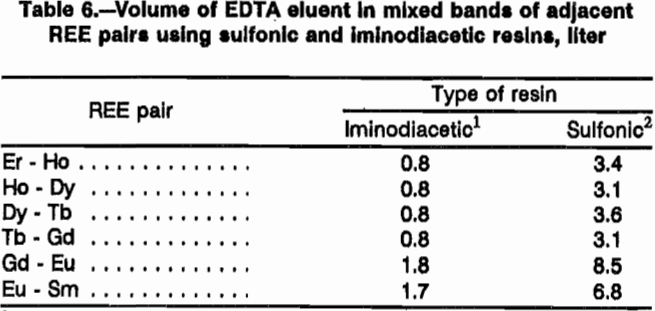
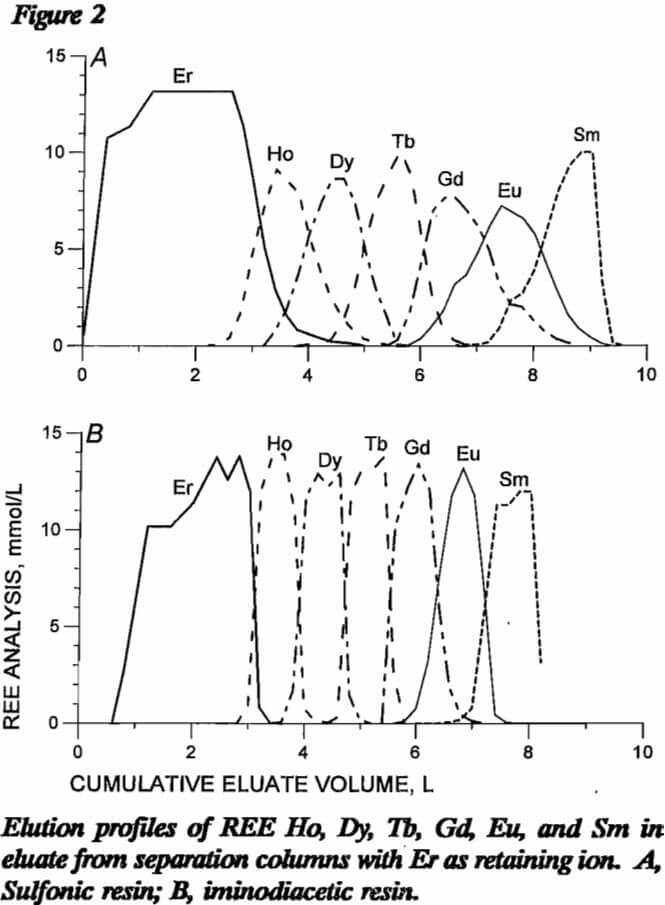
In the last two resin comparison experiments, the loading column of sulfonic resin was prepared as before except 10 mmol each of Tm, Er, Ho, Dy, Y, Tb, and Gd were loaded onto the resin. With sulfonic resin in the separation column, 43 mmol Yb retaining ion was loaded onto the resin; with iminodiacetic resin in the separation column, 26 mmol Yb was loaded onto H form resin. Figure 3 presents elution profiles for this comparative experiment. Note that Y and Dy elute close together in this system and will need further separation. As in the first comparative experiment, iminodiacetic resin yields much cleaner separations, producing mixed bands containing only two REE instead of three or four as with the sulfonic resin. Since the EDTA carries more REE in an iminodiacetic-resin system than in a sulfonic system, less eluent is required. Also, the separations in figure 3 show a better separation using less retaining ion with the iminodiacetic-resin separation column. Iminodiacetic resin is the preferred resin for the separation column.
Column Geometry
Because the REE separation system has two distinct parts, a loading column and a separation column, the
geometries of these columns were considered separately. Traditionally, column length is much greater than column diameter.
Loading Column
The main consideration in determining loading-column size is providing a resin volume that will contain sufficient mixed REE to make the operation economically feasible. Any separation occurring in the loading column is a bonus, not a prime consideration.
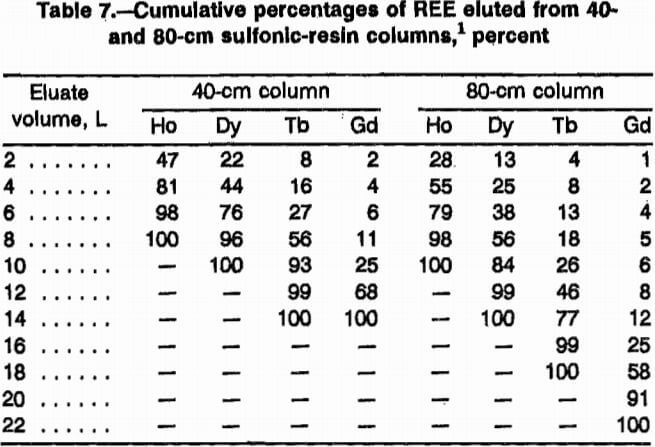
Elution data of REE exiting 40-cm and 80-cm sulfonic-resin loading columns were calculated from test results and are presented in table 7. The 40-cm column was loaded with 32 mmol each of Ho, Dy, Tb, and Gd; the 80-cm column was loaded with 66 mmol each of these four REE. Some general conclusions can be drawn from the results. The 40-cm column required 14 L of pH 6 EDTA to elute all of the REE, while the 80-cm column required 22 L of pH 8.4 EDTA. It is significant that all the Ho was eluted from the 80-cm column in only 9.5 L (i.e., 43% of the total elution) and the 40-cm column required 8.0 L (i.e., 57% of total elution). The Ho was concentrated into a small percentage of the eluent and this will make the relative size of the required separation column smaller. With a smaller fraction of lighter REE eluting with the Ho, a separation column with relatively fewer exchange sites will be required. Length of loading column was the cause of this result.
Note.—Dash indicates sample content was below detection limits.
Separation Column
The separation column is subdivided into two segments. Both segments contain iminodiacetic resin; but the first has primarily NH4 form resin and the second has primarily H form resin. Lengths of these two segments were considered separately with relative segment lengths depending on the size of the loading column and the composition of the mixed REE. NH4 form resin serves two functions: (1) lighter REE eluting from the loading column is collected and held while the heavier REE is eluted from the loading column, thus preventing bleed through of lighter REE; and (2) the partial separation of REE occurring in the loading column is improved. H form resin then completes the separation of REE with an overall smaller amount of retaining ion than could be achieved with NH4 form resin alone.
NH4 Form Resin
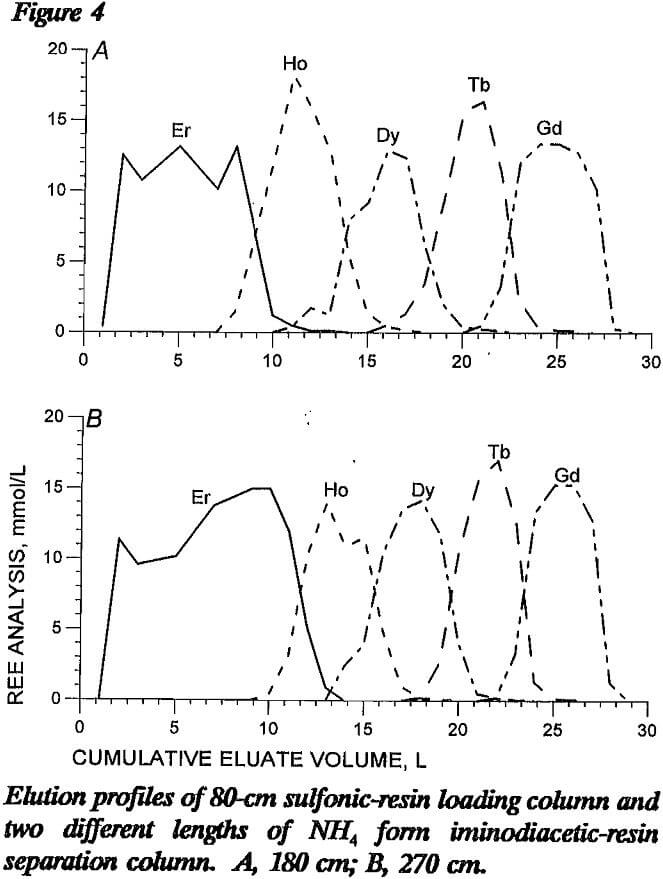
The advantage of using an NH4 form separation column is that the greater loading capacity provides disengagement sites for the mixed REE eluted from the loading column. Iminodiacetic resin has wide range of loading capacities, depending on ionic form and conditioning: equilibrated with pH 8.3 NH4 EDTA, the loading capacity of REE is 0.2 mmol/cm³; conditioned with HCl, the loading capacity is 0.02 mmol/cm³. Figure 4 presents elution profiles from tests with 180- and 270-cm lengths of NH4 form iminodiacetic-resin separation column following an 80-cm sulfonic-resin loading column. In these tests, the separation column did not include a H form resin segment, and the 270-cm column contained proportionately more Er retaining ion. As shown in the figure, REE separation with these two lengths of separation column differed in that the 270-cm column produced better separation of the elution bands with less tailing. Even with the greater amount of retaining ion in the longer column, the mixed- REE band widths were fairly wide.
A short NH4 form column is effective in performing gross separations but further refinement in REE separation is needed to minimize the amount of mixed-REE solutions to be recycled. Although a longer NH4 form segment would undoubtedly narrow the width of the mixed- REE bands, proportionally larger amounts of retaining ion would be required. This tends to nullify any advantage gained by narrowing the mixed-REE band width.
H Form Resin
Using a H form iminodiacetic resin segment following the NH4 form segment offers a way to narrow the width of
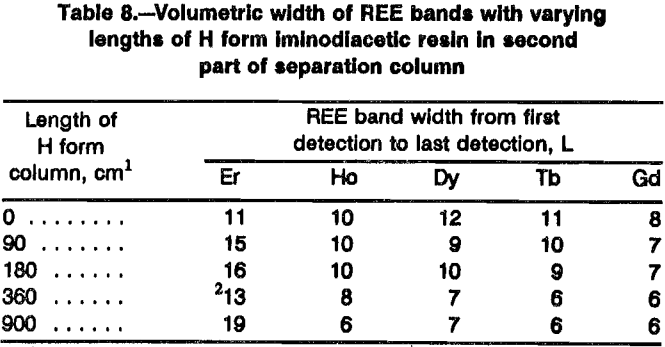
the mixed-REE bands without using vast amounts of retaining ion. Figure 5 presents elution profiles from tests with different lengths of H form iminodiacetic resin fol-lowing 80 cm of sulfonic resin in the loading column and 180 cm of NH4 form iminodiacetic resin in the first segment of the separation column. Inspection of this figure shows decreasing band width and less tailing for each REE as the H form segment of the separation column becomes longer. Table 8 shows the width of individual REE bands produced by various lengths of H form resin columns. Increases in H form resin-column length decreased the individual REE band width. The test depicted in curve D of figure 5 had a H form column twice as long as the NH4 form column and yielded 68% of the eluted REE in fractions with 99% purity of a single REE. The test depicted in curve E had a H form column five times as long as the NH4 form column and yielded between 80 and 83% of the eluted REE in fractions with 99% single-REE purity. Selection of the optimum mix of NH4 form and H form column lengths will depend on the size of the loading column and on the relative concentrations of REE in the feed solution.
Flow Rate
Linear flow rates of eluent solution through the columns were varied to determine to what extent separation was dependent on this parameter. Both loading and separation columns were studied. Flow rates are reported on an empty-column basis, i.e., the volumetric flow rate was measured and converted to a linear flow rate assuming the column contained no resin.
Loading Column
Tests were conducted in the 80-cm sulfonic-resin loading column containing 70 mmol each of Ho, Dy, Tb, and
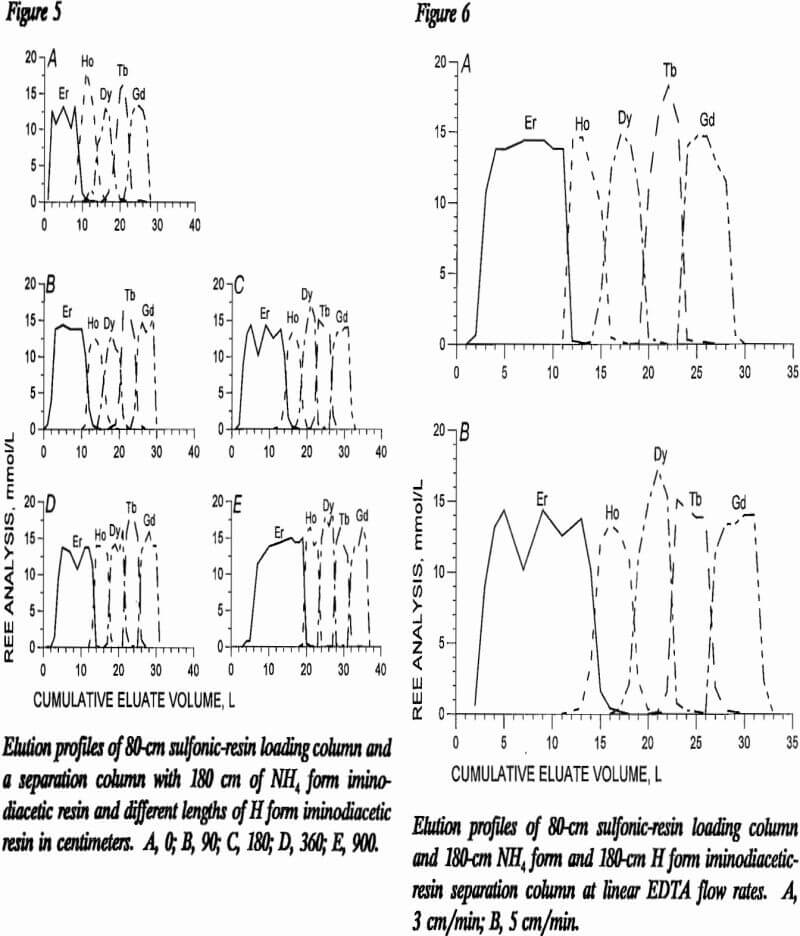
Gd. EDTA eluent buffered to pH 8.4 was passed through the resin at linear flow rates of 3 and 5 cm/min. Eluate was collected in sequential 500-mL volumes and analyzed for the four REE. Eluate profiles for the two flow rates were nearly identical, as shown in table 9, which presents analytical values for the even 2-L samples. These data show that initial separation with the sulfonic resin is independent of flow rate within the range tested.
Separation Column
The same two linear flow rates, 3 and 5 cm/min, were studied in the separation column using a combination of 180 cm of NH4 form and 180 cm of H form iminodiacetic resin. Figure 6, which presents elution profiles generated with these two flow rates, shows that band widths of the various REE are almost identical in these two profiles, but it is significant to note that less tailing was achieved at the 3-cm/min linear flow rate. With additional H form column length, the tailing between REE bands was reduced at the 5-cm/min flow rate, as in figure 5. To increase production rates, the 5-cm/min flow rate was selected as preferential.
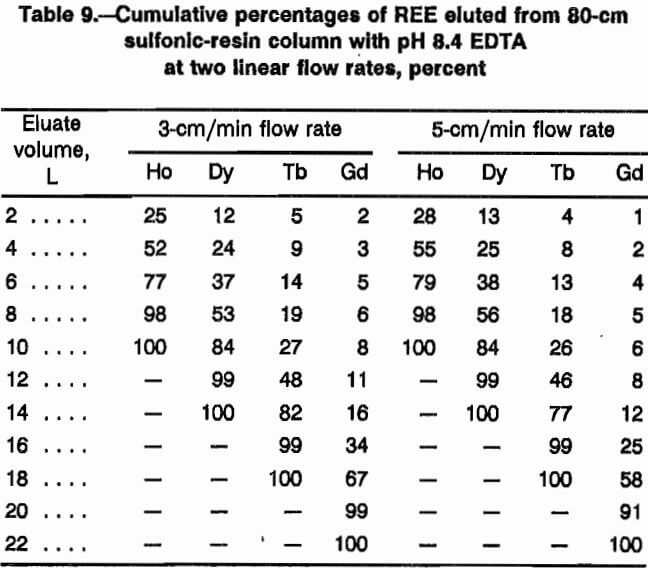
Temperature
A series of two tests helped show REE separation effects of elution temperature. Temperatures of 60 °C and 85 °C were maintained in both the loading and separation columns for these tests. In both tests, the sulfonic-resin loading column was 80 cm in length and the separation column consisted of 180 cm of NH4 form iminodiacetic resin and 360 cm of H form iminodiacetic resin. Other test conditions included a 4-g/L EDTA eluent at pH 8.3 and a linear flow rate of 5 cm/min. Four REE, Ho, Dy, Tb, and Gd, were loaded onto sulfonic resin and eluted with EDTA solution through iminodiacetic separation columns.
Figure 7 shows elution profiles with cumulative eluate volume plotted against REE concentration in the eluate in millimole per liter. The test at 85 °C was terminated before Gd had completely eluted from the separation column; therefore, the Gd curve does not come back to the baseline. The automatic sampler randomly divides the eluent samples into 500-mL portions. With this randomness of sample collection, the profiles are similar although the mixing of elements between separated bands may be less with the higher temperature, perhaps because of increased mass transfer rates. Higher temperatures are, however, more expensive to maintain; therefore, 60 °C was chosen as the preferred column temperature. The literature reports using higher temperatures when H was used as retaining ion. The high temperature prevents H4EDTA from precipitating in the resin. High-pressure columns are required to prevent high-temperature eluent from boiling.
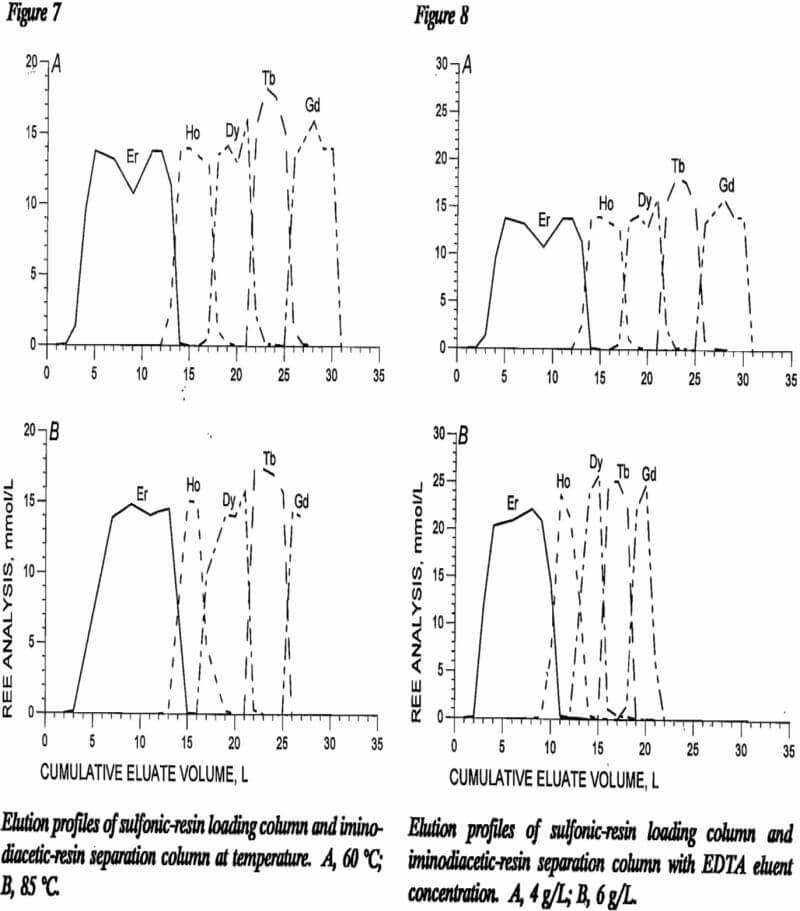
EDTA Concentration
Two different EDTA concentrations were tested in this study: 4 g/L and 6 g/L. Figure 8 shows elution profiles from one test at each of these concentrations. Band widths of separated REE are narrower with 6 g/L EDTA, as would be expected with 50% more REE carrying capacity; but the mixed bands are wider than with 4-g/L EDTA solution. A longer separation column would be required to contain the larger mixed-REE bands needing recycle. Any individual REE having a low concentration in the mixed feed solution would be difficult to recover without either an extremely large system or a large recycle of mixed REE. The preferred concentration from this study was 4 g/L EDTA.
pH
Eluent pH has one of the more complex effects investigated in this study. EDTA with a pH less than 8 introduces two complications to the system: the number of resin exchange sites occupied by REE in the separation column can be reduced during an elution, and the EDTA eluent does not carry enough NH4 to regenerate the NH4 form iminodiacetic resin.
The amount of REE carried by EDTA from a sulfonic resin depends on the concentration of NH4 associated with the EDTA (table 10). For iminodiacetic resin, each mole of EDTA carries 1 mol of REE and is independent of pH. For pH 8.5 NH4 EDTA, equations A and B show that each mole of EDTA carries more moles of REE from iminodiacetic resin (i.e., 1.0) than from sulfonic resin (i.e., 0.8). The consequence of this is that EDTA removes REE from the iminodiacetic separation column faster than REE is carried in from the loading column. With low-pH-EDTA solutions (i.e., pH 6 and lower), the depletion of REE from the separation column is significant. Since this system uses a separation column with relatively few exchange sites (i.e., about half as many sites in the separation column as loading column), the separation column can be completely depleted of REE before all the REE has been eluted from the loading column. This loss of exchange sites is negated to some degree by the fact that fewer exchange sites are required at the end of the REE separation because fewer species of REE remain to be separated. Also, exchange site depletion occurs to the NH4 form resin first, which is less detrimental. For this reason, a pH above 8 is desirable.
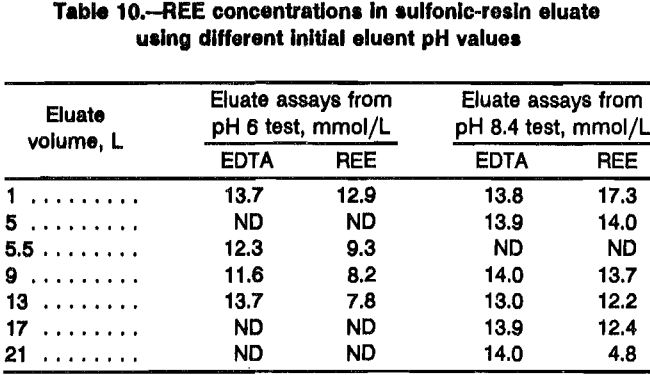
Ammonium adsorbed on the iminodiacetic resin during eiution of the REE conditions the resin into the NH4 form for the next cycle of loading the retaining ion. Each mole of REE replaces 3 mol of NH4 when REE retaining ion is adsorbed on NH4 form iminodiacetic resin, and each mole of REE replaces about 1 mol of NH4 and 2 mol of H when REE is adsorbed to H form resin. As stated above, each mole of EDTA carries 2 mol of NH4 when it exits the NH4 form resin and 1 mol of NH4 after it elutes 1 mole of REE from the H form resin (see reaction B). If the separation column contains 1 mol of REE adsorbed on H form resin and 1 mol adsorbed on NH4 form resin, then 2 mol of EDTA are required to elute the REE. To regenerate the resin so as to load 2 mol of REE retaining ion, 3 mol of NH4 is adsorbed on the NH4 resin, 1 mol of NH4 to the H form resin, and 1 mole of NH4 for each EDTA eluted through the separation column. Based on this calculation, each EDTA requires three NH4 to regen-erate the resin. Low pH EDTA (i.e., pH 6 has 2.3 mol of NH4 with each mole of EDTA) conditions only a fraction of the iminodiacetic resin into the NH4 form before all the REE is eluted. With pH 8.5 EDTA (i.e., 3.3 mol of NH4 per mole of EDTA), the concentration of NH4 is high enough to condition the resin. The preferred EDTA eluent pH is between 8 and 8.4. This finding corresponds to that of past researchers, but for additional reasons.
Recycle of Mixed REE
Between each band of separated REE exiting the column is a mixed band of the two adjacent REE. By recycling this mixture to the columns during the next elution cycle, overall recovery in the next cycle is increased. In essence, the mixed bands replace themselves during the next cycle, such that all of the REE loaded onto the loading column in the next cycle is eluted as separated products. Direct recycle has at least three advantages in REE processing:
- Repeat treatment of mixed-REE-EDTA complexes is avoided.
- Mixed-REE-EDTA complexes are binary mixtures only, not mixtures of several REE; therefore, they do not significantly add to the mixture of REE eluted from the loading column. By recycling the REE binary mixtures through the column in the order of heaviest to lightest, these binary mixtures effectively increase REE processing capacity by putting more REE into the separation column without actually lengthening the loading column.
- The binary-REE mixture displaces only heavier REE as it elutes through the separation column. Thus, recycle solution has a significant separation effect as it passes through the mixed REE adsorbed on iminodiacetic resin in the first part of the separation column.
A column elution test was conducted with binary-mixture eluate recycle to determine process feasibility. The 160-cm-long loading column in this test contained about 130 mmol of each of four REE, Ho, Dy, Tb, and Gd, loaded onto sulfonic resin. The separation column had a total length of 900 cm, 360 cm as NH4 form and 540 cm as H form iminodiacetic resin, and was loaded with about 268 mmol Er retaining ion. After the columns were loaded and rinsed, 2 L of pH 8.4 EDTA was passed through both loading and separation columns at 5 cm/min to replace the rinse water. This was followed by 1.5 L of each of the following binary-REE-EDTA mixtures: Er and Ho, Ho and Dy, Dy and Tb, and Tb and Gd. Elution with fresh pH 8.4 EDTA solution was then resumed until the separation column was clear of REE.
Figure 9 shows the elution profile of this recycle test. Some changes in REE elution from the iminodiacetic resin were noted in that the REE-EDTA complex did not accumulate on the resin as much in the first part of the elution as in other tests. The elution mechanism for eluting REE from sulfonic resin was probably also disrupted in that REE-EDTA complexes from iminodiacetic-resin elution, not fresh EDTA eluent, were being passed through the sulfonic resin. This may account for the wide fluctuations in eluate EDTA and Er concentrations during the recycle period. Other figures in this report show fluctuations of REE concentrations due to analytical precision, but the EDTA analytical results did not. The REE concentrations for figure 9 show broader bands of separated REE exiting the column than do profiles from other tests with the same number of REE moles saturated on the loading column. Analysis of the curves in this figure show that the percentages of REE recovered in eluted fractions with 99% single-REE purity varied from 71% of the Ho to all of the Gd. This wide variation illustrates the disruption that occurred early in the elution, but it also shows that recycle of the mixed bands directly without any intervening treatment is feasible. The volume of mixed bands produced in this recycle test was comparable to the volume produced in previous tests employing no solution recycle, again
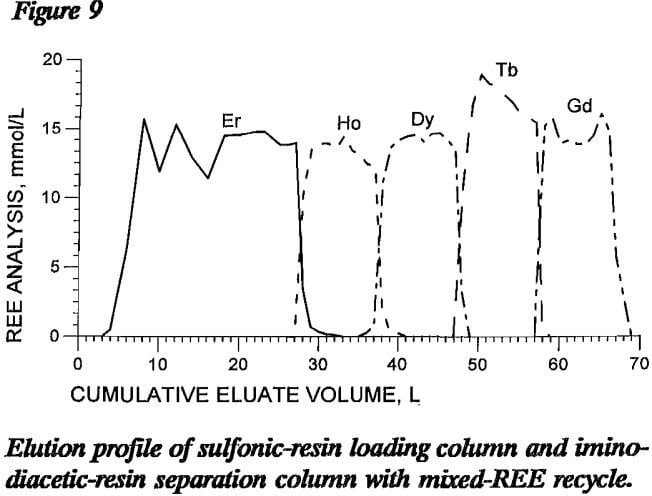
indicating that the recycled REE were separated during the elution cycle.
If the recycle performs as expected, mixed-REE bands will regenerate themselves for recycle to the next elution cycle; and all of the REE loaded onto the sulfonic resin in the loading column will report in the eluate as separated REE products. The preferred process includes mixed-band recycle to the separation column only, not to the loading column, as was done in the preliminary test. Stopping eluent flow through the loading column during mixed-band recycle in the separation column is not expected to cause complications in the elution. Tests showed that stopping the elution overnight had little effect on the results of the separation. Additional test work should be done to obtain definitive data for designing the recycle system as to the point of insertion for the recycled solutions and the timing of insertion.
Separation of REE and EDTA
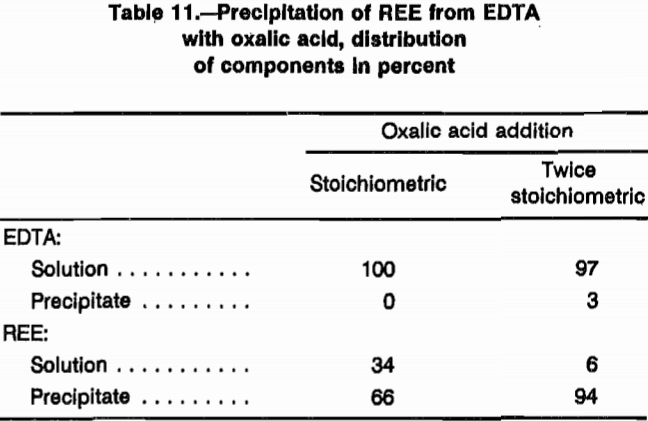
Part of the separation and recovery process for REE is production of a marketable REO. Comprehensive testing was not conducted in this area; however, EDTA precipitation with HCl and REE precipitation with oxalic (C2O4H2) acid were examined in feasibility tests.
HCl Precipitation of EDTA
Recovery of EDTA for recycle within the process was demonstrated by adjusting the pH of separated Ho EDTA solution from one of the ion-exchange tests. The laboratory test used concentrated HCl to adjust the pH to 1.0; but for ease of acid handling, pH adjustment with 6N (normal solution) HCl may be preferable. After sitting overnight, the pH-adjusted solution was filtered and the precipitate was washed with water. H4EDTA recovery in solid form was 92% with neither Ho nor Cl2 reporting to the precipitate. This precipitate would be used to prepare more EDTA eluent.
This procedure could also facilitate recycle of the Er retaining ion. Erbium chloride solution recovered from the first eluate exiting the column represents the eluted retaining ion. This dilute solution can be used to reload retaining ion onto the separation column for the next elution cycle.
REE-Oxalate Precipitation and Roasting
Because REE-oxalate compounds are insoluble in most aqueous solutions, precipitation of these compounds from the REE-EDTA solutions was proposed as an approach for recovering purified REE. During one of the column ion-exchange tests, 200-mL samples of Ho-and Dy-bearing eluate were treated with oxalic acid at two addition ratios: stoichiometric and twice stoichiometric. On a mole ratio basis, these additions were 1.5 mol oxalic acid to 1 mol REE and 3 mol oxalic acid to 1 mol REE. Table 11 presents data from these precipitation tests. Twice the stoichiometric addition of oxalic acid precipitated 94% of the REE from the EDTA solution.
Summary
In one configuration of the USBM ion-exchange system, over 80% of the eluted REE was recovered as fractions with 99% purity of each element; and with recycle, this recovery percentage was increased to around 90% of the eluted REE. These recovery figures for separated REE are especially good in view of the size of the columns used in this research. Additional research would be invaluable in better demonstrating mixed-band recycle in the process, in more definitively determining elution mechanisms from each of the resins, and in applying this technology to actual REE process solutions. Completion of the current USBM ion-exchange research has improved REE separation technology. The USBM method is different in the following areas:
- An ion-exchange column consisting of two sections, a loading section and a separation section, provides both high levels of REE loading and good REE separation. A different resin is used in each section: sulfonic resin in the loading section to provide high levels of REE loading and iminodiacetic resin in the separation section to provide good separation of the REE.
- The separation section is further divided into two segments: one with NH4 form iminodiacetic resin to provide higher storage for, and further partial separation of, mixed REE eluted from the loading section, followed by one with H form iminodiacetic resin to provide final REE separation.
- Erbium is used as the retaining ion in the separation column rather than copper, zinc, or hydrogen as has been used extensively in the past. Bleed through of heavier REE elements was prevented; recovery and recycle of the Er retaining ion can be accomplished easily; and a temperature of 60 °C was satisfactory for the process. Because Er is one of the elements to be separated in the matrix, using it as a retaining ion does not introduce contaminating substances into the process.
- In comparison tests with displacement chromatography using sulfonic resin and EDTA, selective elution of REE from iminodiacetic resin demonstrated more effective separation of REE Yb through Sm. The volume of mixed-REE bands between the separated bands was less with selective elution, and fewer exchange sites and hence less EDTA eluent were required to effect the separation.
- Processing time was shortened dramatically. Previous processing times that ranged up to several months have been shortened to approximately 2 weeks.
- EDTA solutions with binary REE are recycled to the ion-exchange system to increase recovery. Also, reagents are recycled more efficiently.
