So far, no account has been taken of the loss of gold which is contained in pyrites, as it has been assumed that these are saved by concentration if they are valuable, and this subject is dealt with in earlier. Nevertheless, as this gold comes under the head of non-amalgamable gold, its physical state and the causes of its disinclination to unite with mercury may conveniently be considered here. In general, pyrites yields only a small proportion of its gold contents if it is run over the amalgamated plates, and if it is ground very fine in a pan with mercury the percentage extraction is better. Among the old processes used for the amalgamation of the gold in pyrites may be mentioned the treatment in revolving wooden barrels with mercury, as practiced at the St. John del Hey Mine, and the practice of leaving the pyrites to be decomposed by weathering before grinding it with mercury. This method of oxidation seems to be decidedly inferior to the alternative plan of roasting the sulphides, by which the oxidation is rendered more complete and the particles of gold agglomerated to some extent. However, the amalgamation of pyrites, even when roasted, is far from perfect, part of the gold still remaining in a condition unfit for extraction in this way. The ores, which have been met with in various parts of the world, consisting mainly of limonite or hydrated oxide of iron, and in most cases believed to be the result of decomposition of pyritic ores by atmospheric agencies, are also extremely refractory, causing the mercury to sicken rapidly, and yielding only about the same percentage of gold as can be obtained from unoxidised pyrites.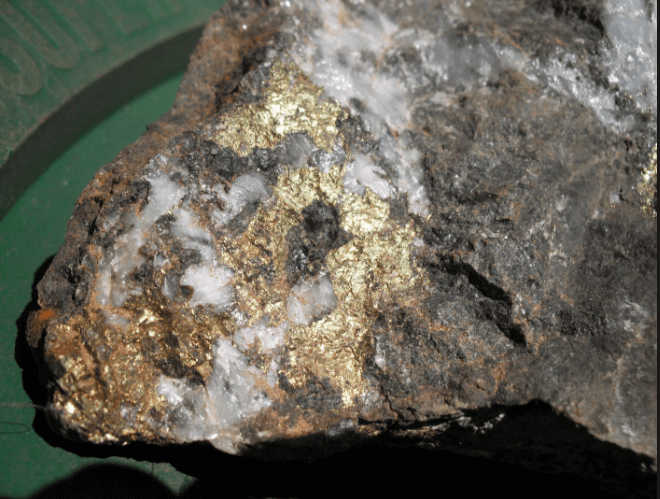
The most celebrated case of this kind is that of the surface ore at Mount Morgan, in Queensland, which was an ironstone gossan consisting of siliceous brown iron ore, derived according to one view from the decomposition of pyrites. Although the gold appeared to be free, it could not be amalgamated, yielding only about 30 per cent, when crushed in batteries and subjected to prolonged grinding in pans with mercury. When the ore was dehydrated by roasting in reverberatory furnaces the extremely fine particles of gold were agglomerated, and between 80 and 90 per cent, could then be extracted by amalgamation, the remainder being presumably coated by oxides of iron. The richness of the ore, however, made even this result unsatisfactory, and a process of chlorination was adopted in practice. A similar case was noticed by Mactear in South America, where a limonite ore which only yielded from 35 to 40 per cent, of its gold when treated in Huntington pans, was made to yield between 85 and 90 per cent, by merely subjecting it to a dehydrating calcination before amalgamating it. Louis Janin, Jr., mentions another case in the ores of the Southern Cross Mine, Deer Lodge County, Montana, which consist of limonite derived from the alteration of pyrites. In panning large samples, only one or two specks of gold could be seen, although the ore contained from 1 to 2 ounces per ton. This ore yielded only about 40 per cent, on being amalgamated, but over 90 per cent, was dissolved out by leaching the raw ore with cyanide of potassium, and similar results were obtained by chlorination. Here the ore was thoroughly decomposed, but yet the gold would not amalgamate to a much greater extent than if it were still contained in the original pyrites, whilst the chemicals at once dissolved it.
https://www.911metallurgist.com/pyrite-concentrate-gold-leaching/
The balance of evidence, however, seems to be in favour of the theory that gold exists in pyrites in the metallic state. Although the metal is generally invisible in undecomposed crystals of pyrites, it becomes visible when such crystals are oxidised either by air and water in nature, or by means of nitric acid, or by being roasted or subjected to deflagration with nitre. As a result of such decomposition, particles of bright lustrous gold, angular and ragged in shape, but of considerable size, often become apparent. These particles may be separated from the oxides of iron by washing, and the use of nitric acid, followed by panning, is frequently resorted to in order to detect gold in pyrites. Moreover, although usually invisible, gold can sometimes be seen in unroasted pyrites. As long ago as the year 1874, Richard Daintree and Latta found specimens of cubical pyrites, in which gold could be seen under a microscope gilding the cleavage planes of the crystals. Again, G. Melville- Attwood, on examining crystals of auriferous pyrites from California in 1881, found that the faces of the crystals were gilded in some places, and that here and there little specks or drops of gold occurred, partially imbedded in the pyrites. These films were too thin to be detected by an ordinary lens, so that it did not seem surprising that such impalpable material could not be taken up by mercury. Louis Janin, Jr., more recently found crystals of pyrites in a porphyritic gangue from the Republic of Colombia, which had gold in small globules on their surfaces. Lastly, it has long been known that crystals of pyrites are often found adhering to an amalgamated plate, the particles of gold on their surfaces having been amalgamated. It seems likely, in view of all these facts, that most if not all of the gold is in the metallic state, and its occasional refusal to amalgamate is not very surprising, when it is remembered how completely a thin coating of certain sulphurised compounds prevents amalgamation, and how readily sulphuretted hydrogen would be evolved from decomposing pyrites. Some authorities have contended that the metallic gold is disseminated mechanically through the mass of pyrites, but the action of potassium cyanide, in dissolving the whole of the gold out of comparatively coarsely crushed pyrites, seems to point to the correctness of the view that the interior of the crystal is not auriferous, the deposition of the gold being superficial, so that the enrichment of the pyrites is confined to its crystalline faces, and possibly, but not probably, to its cleavage planes. Strong evidence of the richness of the outside of the crystals of pyrites in the Banket ore of the Transvaal is given by Caldecott in his papers on the treatment of ore in the Transvaal.
The following details of a microscopical examination by Prof. Morton, of the condition in which pyrites is left after being leached with cyanide, confirms this view to some extent:
“Upon the ordinary auriferous sulphide of iron, or arsenical pyrites, the solution of potassium cyanide acts readily, not by dissolving the sulphuret, but by attacking the gold upon its exposed edges, and eating its way into the cubes by a slow advance, dissolving out the gold as it goes. An examination with the microscope of the pyrites after the gold has been removed, suggests the method of the operation. A sample of very rich pyrites from a mine north of Redding, was treated with a weak solution, containing less than two-tenths of 1 percent, of cyanide, for 168 hours; the assay showed a complete extraction of the gold; as the sulphurets showed no change in their appearance to the naked eye, some of them were placed under the microscope.
“ There is no change visible in the form of the crystals as a whole; along the fractured faces the mispickel looks clean and unaltered, showing the silvery-white colour and intense refraction of the arseno-pyrite. Upon the faces of the crystals appear dark lines, short, and parallel to each other. In places they are crowded close together; in other parts they are at considerable distances, but always in parallel lines. The lines vary in length, being from four or five to over a hundred times their width ; the lines are very irregular and often broken. These lines are fissures in the pyrites, and extend so deep into it that the microscope does not reveal their depth. By using the higher powers the walls of one of the fissures were seen to be completely honeycombed, looking somewhat like two empty honeycombs set opposite each other; evidently, the mineral removed was crystallised along its contact walls at least. As the raw or untreated pyrites does not show any such fissuring, but, upon the contrary, shows a surface marked only by striation lines common to pyrites, I assume that the fissuring in the treated sample is caused by the solution acting upon some soluble mineral, probably gold, arranged in plates, occurring in groups, but which, by its colour and isomorphism and the extreme tenuity of its lines, is indistinguishable from the mass of pyrites enclosing it.”
