Table of Contents
The secondary metals industry has been a major segment of the domestic economy for years. Many secondary commodities (heavy melting steel and lead storage batteries are familiar examples) have become so well established and recycled so promptly that industry commitments for metal would be impossible without them. Although some recovery and refining processes developed for primary metals are also used in scrap, the technology on secondary metal reclamation has lagged.
There are a number of reasons for this lag. Difficulties in collection, variability of materials, and rapid obsolescence of fabricated products are several of the factors contributing to the mounting accumulation of solid waste. The situation is aggravated by the growing complexity of modern scientific. and engineering technology. The components of highly sophisticated instruments, apparatus, and equipment are often intricate composites of several metals and nonmetals, which are almost impossible to reclaim from worn items.
Processing economics is usually the sole consideration in determining whether to recover secondary metals from particular scrap commodities. This may make economic sense at the moment, but sociologic and ecological implications could be paramount in the future. Increasing public awareness of conservation and the consequences of land pollution has generated pressure on industry and the Government to find acceptable means of disposing of or recycling solid wastes.
Because of the unique combination of physical properties possessed by iron and copper, they are compatible for fabrication into various types of electrical products. Because the copper has high electrical conductivity and ductility and the iron has high mechanical and ferromagnetic strength, they can be fabricated into intimate mechanical composites such as motor armatures, field cores, transformers, copper-clad wire, etc. After the useful service life of these items has been expended, recovery of the iron and copper, as separate fractions, presents a problem. Both the clean high-silicon steel and the copper have relatively high scrap values, but each material is a deleterious contaminant of the other. As such, a clean separation of both materials is not presently possible except by tedious and/or slow methods.
This Bureau of Mines report presents the results of research on the separation and recovery of copper and steel from scrap items in which substantial percentages of both metals are present. There are several procedures by means of which this can be done, some of which are practiced commercially. These procedures include leaching, mechanical concentrations such as crushing and magnetic separation, and disassembling by hand labor. Leaching is an efficient means of extracting copper but is economical only on a large scale. The other procedures may also be costly or suffer from shortcomings in recovery or grade of product. An excellent compilation of these and other methods for producing secondary copper has been prepared by Spendlove.
The Bureau has developed a method for reclaiming the components of copper-iron scrap items, armatures, some electronic scrap, and gilding metal; details were published in 1968. Continued research has resulted in a significant improvement of separating efficiency, particularly for armatures.
Acknowledgments
The authors wish to cite the Economic Evaluation Group, Salt Lake City Metallurgy Research Center, Salt Lake City, Utah, for their economic evaluation. Figures 5-7 were provided through the courtesy of A. F. Holden Cos, Milford, Mich.
Appendix B was provided through the courtesy of Upton Industries, Inc., Roseville, Mich.
Laboratory Evaluation
Experimental Procedure
Preferential melting would seem to be an appropriate means for separating copper and steel considering the wide spread in melting points (1,083° and about 1,450° C, respectively). Sweating techniques are effective in reclaiming the low-melting-point metals such as lead, tin, zinc, and aluminum and have been commercial for some time. However, experiments in gaseous combustion atmospheres showed ordinary sweating procedures were not satisfactory for the copper-iron system owing to oxidation of iron and entrapment of copper within the oxide scale. A reducing atmosphere diminishes scaling but enhances the tendency for copper to alloy with iron.
In Bureau research, it was discovered that if the sweating is accomplished in a neutral molten substance such as barium chloride, both oxidation and alloying of copper and iron are lessened, and the liquid bath promotes rapid heat transfer. Although this constituted a considerable improvement, the steel core (as in scrap armatures) still retained several percent of copper. Recently, an almost quantitative separation was achieved by pretreatment with chemical reagents that alter and coat the surface of the iron so it is less subject to brazing in the molten bath. In effect, a barrier interface is created on the steel surface to prevent intimate contact of the liquid copper while it flows over the surface of the armature. The exact procedure is as follows: A number of small (1-¾-inch-diameter) armatures were dipped into saturated solutions of sodium sulphate (5 to about 40 percent depending on temperature) or sodium silicate (about 40 to 60 percent) and then dried at about 100° C. The immersion time is not critical. This may also be accomplished by spraying the solution on the scrap or by immersing completely as in a rotary drum.
A graphite crucible containing about 4 pounds of the salt, which comprises the molten separating medium, was placed into the laboratory induction furnace (fig. 1). The furnace was brought up to the selected operating temperature of 1,150°, 1,200°, or 1,250° C as measured with an optical pyrometer. A motor armature, pretreated as previously described, was placed in the molten salt bath with tongs and allowed to attain equilibrium temperature. This usually required about 1 or 2 minutes after which the armature was soaked for the desired period, which ranged from 2 to 15 minutes. Copper droplets are sweated off the scrap and collected in the bottom of the vessel; the liquid copper could be easily tapped from a vessel of commercial size. The armature was then grasped with the tongs and shaken to remove some of the molten copper from the crevices. The armature was removed from the salt bath and dropped onto a hard surface to remove more molten copper from the crevices of the steel core. The salt bath heat transfer medium may be reclaimed and reused many times.
Analytical samples of the steel were made by cutting the armature in half, transversely, and then removing several typical steel laminae in a lathe or by hand (fig, 2). Chemical analyses were performed on the sampled lamellae.
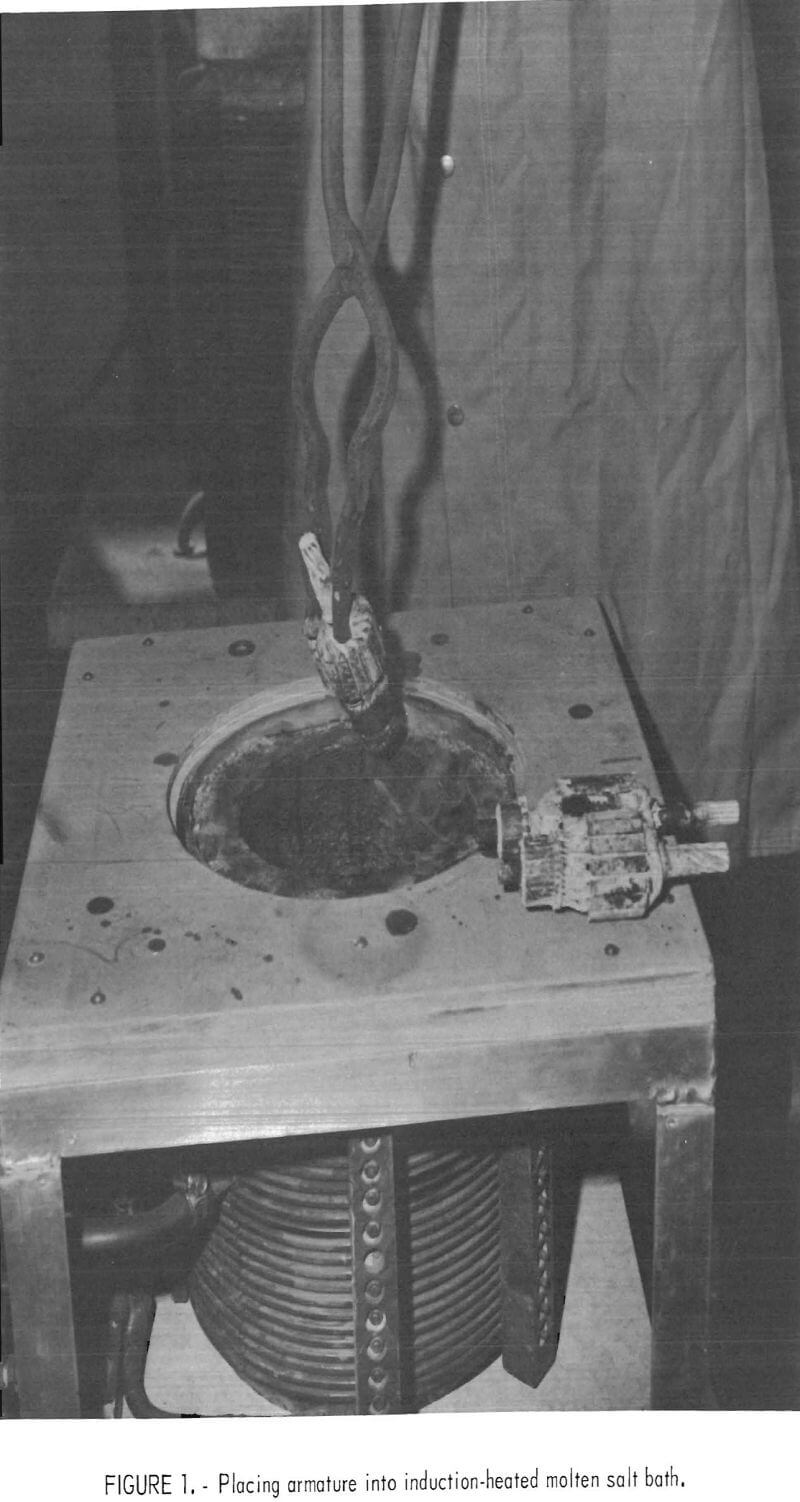
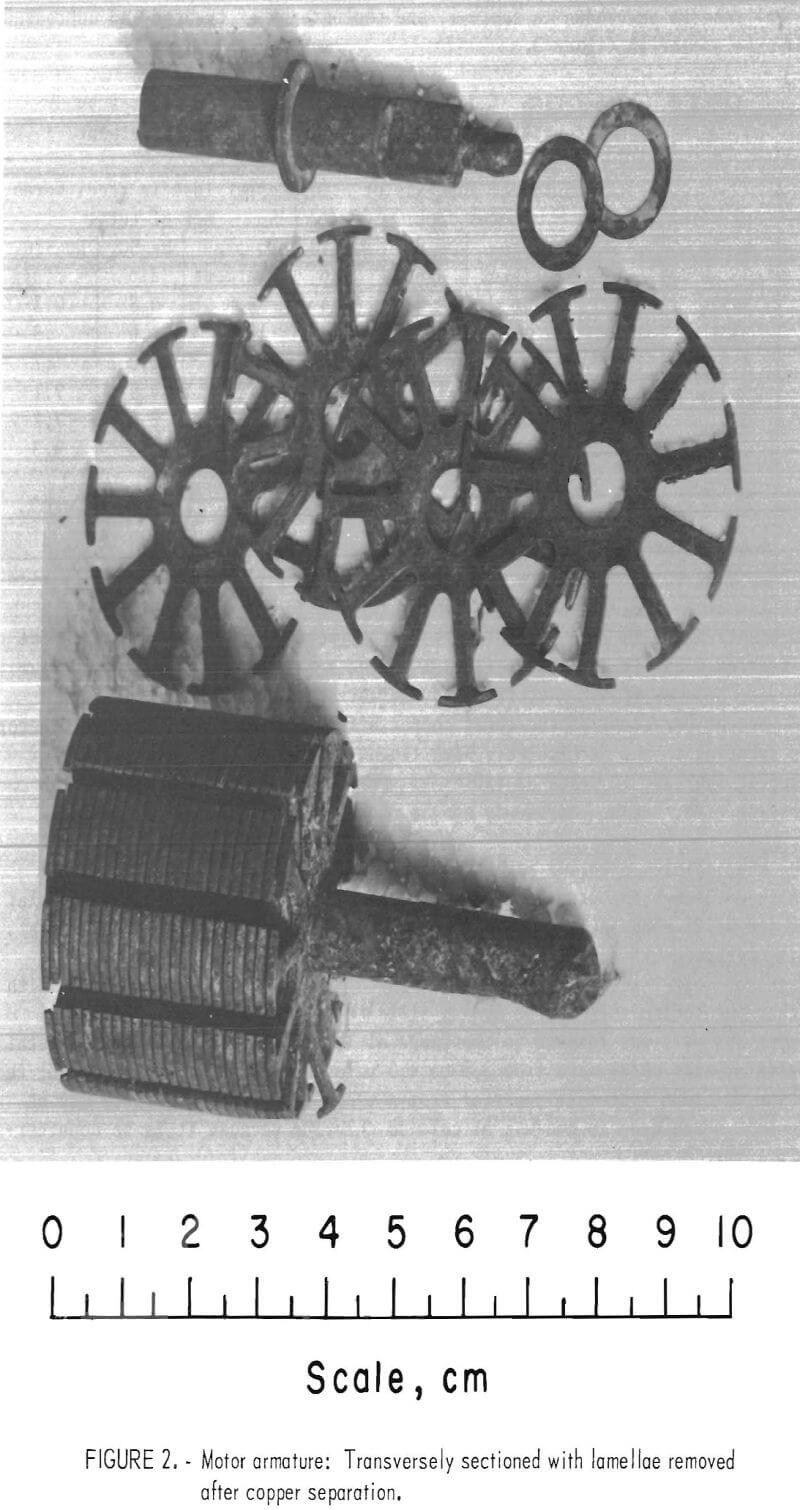
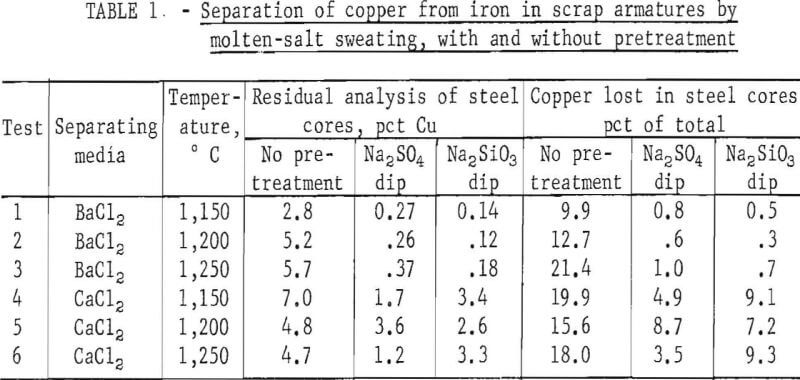
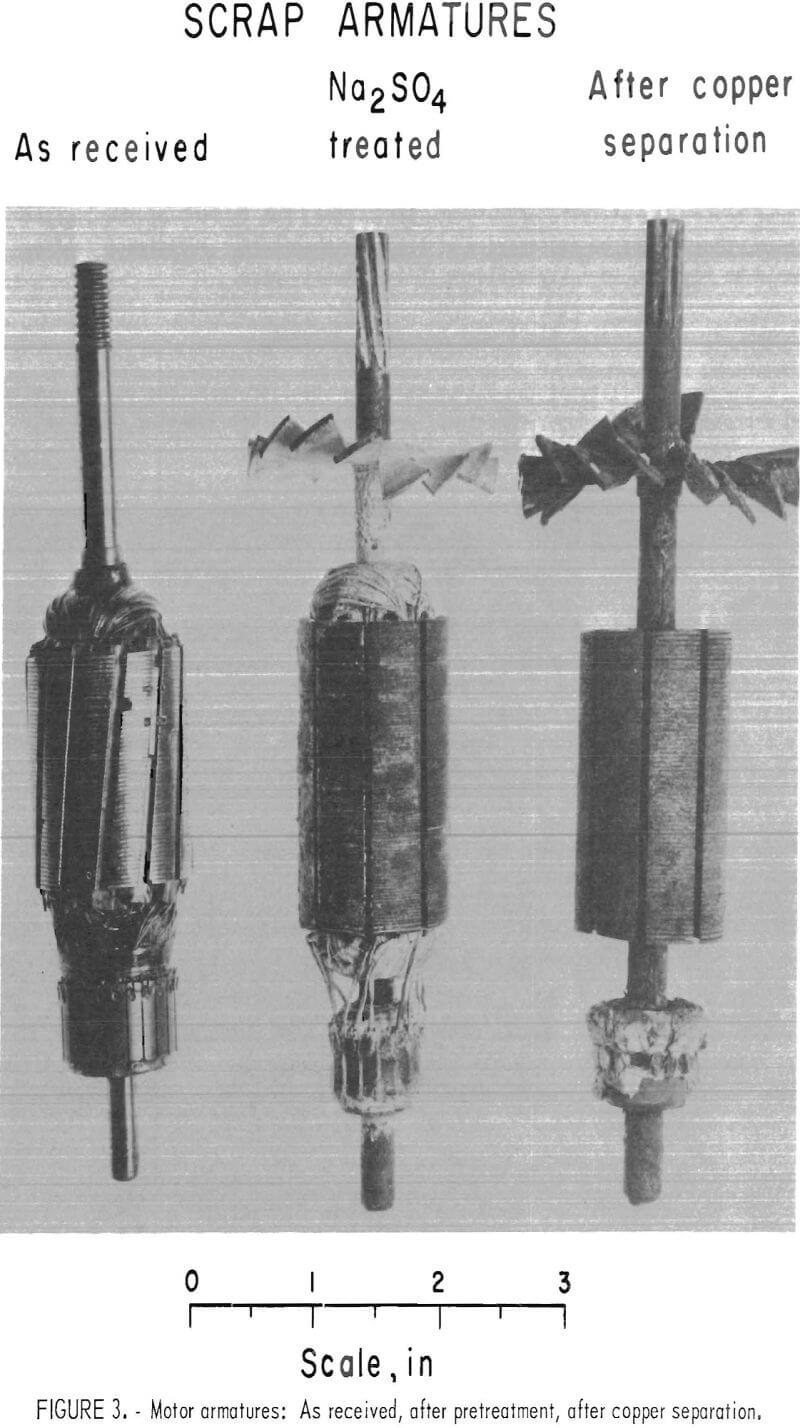
Typical results are shown in table 1, and photographs of treated and untreated armatures appear in figure 3.
Note.-These data were extracted from tables A-1 through A-8 in the appendix.
Analyses of the steel cores (table 1, tests 1-3) for residual copper content show what is possible by this procedure. Separation in molten barium chloride alone yields steel cores containing 2.8 to 5.7 percent copper, which represents a 9.9 to 21.4 percent loss of that metal. When the armatures are pretreated with either Na2SO4 or Na2SiO3 , the separation of copper in barium chloride becomes almost quantitative, and as shown, the losses are only 1.0 percent (or less) of the total copper originally present. The time factor for sweating is not particularly significant (tables A-1 through A-8). For example, the last 10 tests of table A-1 were run with 1 minute increment sweating time, but no dependency can be attributed to the treatment time.
When calcium chloride is employed as the molten medium, the gains realized through the pretreatment (table 1, tests 4-6) are not as spectacular but are still substantial. Note that without pretreatment, the steel cores still retained 4.8 to 7.0 percent copper, depending on temperature. The losses of copper without pretreatment ranged from 15.6 to 19.9 percent. With either the sodium sulphate or sodium silicate pretreatment, the residual copper analysis was lowered to the range of 1.2 to 3.4 percent, and the total losses of that metal were from 3.5 to 9.3 percent. Although it is evident the barium chloride system is superior from a technical viewpoint, there may be economic advantages in the use of calcium chloride because it is so considerably lower in cost.
The typical copper product, which results from the treatment of scrap motor armatures, analyzes 96 percent or more copper, 1 to 4 percent iron, and fractional percentages of lead, zinc, and other elements. This product may be refined by standard refining processes. The resultant iron product may be a suitable raw material for certain cast irons or reinforcing rods.
Sweating With Other Predip Treatments
In addition to the sulphate and silicate predip treatments, a number of other organic and inorganic predip treatments were considered (table A-8).
A listing of these compounds is as follows: Braze inhibitor paint-titanium dioxide pigment, braze inhibitor paint-calcium carbonate pigment, sodium bismuthate, bismuth chloride, vanadium pentoxide, ammonium molybdate, ammonium molybdate-tin chloride, sodium phosphate, sodium borate, and tin chloride. Although most of the resting was concentrated on sulphates and silicates as brazing inhibitors, a limited number of tests were executed with these other active agents. In general, the results do not appear to be as successful as those tests with silicate or sulphate inhibitors, but in view of the limited tests, they cannot be disregarded as brazing inhibitors. Additional testing would be required to fully evaluate their effectiveness.
Electronic Component Sweating
The applicability of the barium chloride sweating technique was evaluated on a broad range of copper-containing materials. Basket sweating tests were performed on copper-base electronic components (fig. 4).
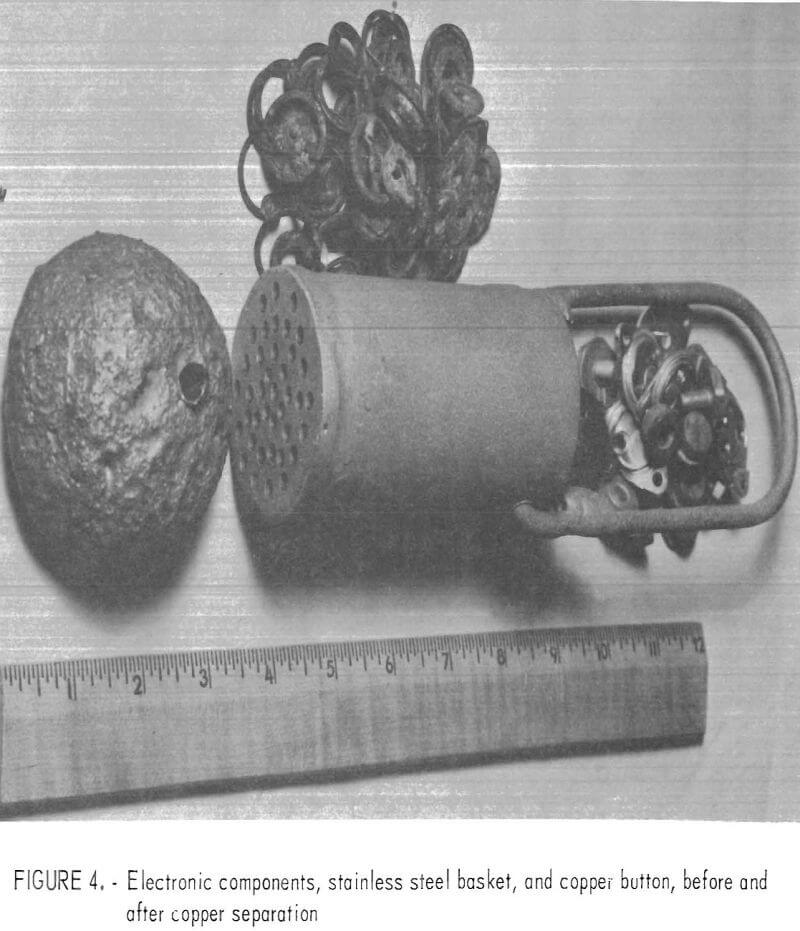
In these experiments, stainless steel baskets were used to sweat several 500-gram lots of mixed components about 5 minutes each at 1,250° C. A composite copper ingot of these batches was produced, which accounted for 85.7 percent of the input weight with 0.9 percent iron as the only objectional impurity.
Discussion and Concluding Remarks
The operation of any sweating separation is based on close temperature control above the melting point of one metal but below that of the other. In the present case, the molten bath not only facilitates temperature control but also improves heat transfer and limits oxidation. The purpose of the pre-treatment is to render the steel less susceptible to alloying with copper either by controlled, mild oxidation (as in the case of sodium sulphate) or by coating the steel surface with a nonreactive substance (as in the case of sodium silicate).
Components of the molten baths were, as noted earlier, anhydrous barium chloride or calcium chloride. Commercial heat-treating salts for high-speed steel were also used successfully; these are proprietary mixtures with barium chloride as a principal component. The baths are not limited to these substances. Components of the molten baths may be any salt, slag, or glass, provided the substance is stable, noncorrosive, relatively inexpensive, possesses a moderately low vapor pressure, and forms a fluid melt in the appropriate temperature range. Similarly, the pretreatment could be effected by other soluble or liquid chemicals, which would render the steel less susceptible to alloying with copper and is compatible with the balance of the system. Care must be taken in the choice of salt mixtures since some combinations are explosive or may emit poisonous gases.
In consideration of the rising demand for copper over the projected future, which is reflected in an escalating price tag recovery of copper from secondary sources should be encouraged. The technique described is a workable means of separating copper and steel from scrap commodities in which these are the major ingredients. There are some limitations on the method that must be recognized. One of these limitations is the copper content of the electrical scrap, which must be rich enough to bear virtually all the processing costs. Another potentially limiting factor is the physical structure of the scrap; it must be sufficiently open to allow the free flow of molten salt and metal and must not be so intricately constructed as to retain substantial quantities of either.
The salt bath sweating process should pose no unusual equipment problems in being scaled up to commercial size. The economics have been evaluated based on a treatment line capable of recovering 1 ton of copper per day. In this model, the scrap copper is presumed to be a byproduct of a fairly large automobile wrecking yard, but the model would be equally appropriate for other sources of worn or reject fractional horsepower motors. The separating vessels would be suspended-electrode, salt-pot furnaces, which could be modified to provide a semicontinuous discharge of copper. These internally heated, electrical resistance furnaces, available from several suppliers
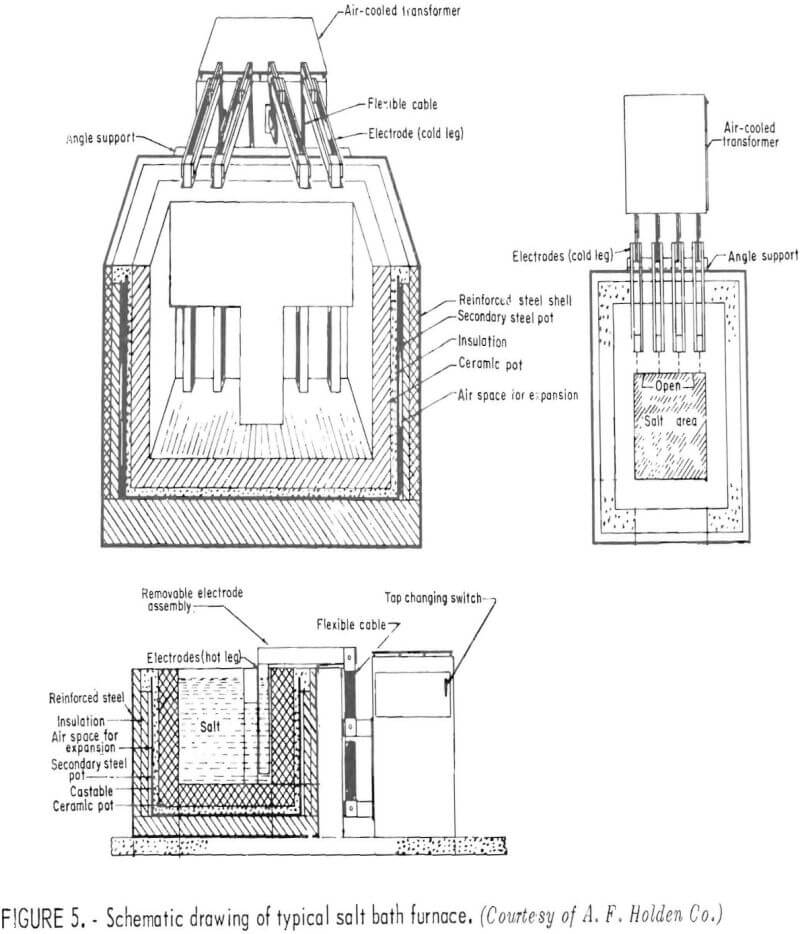
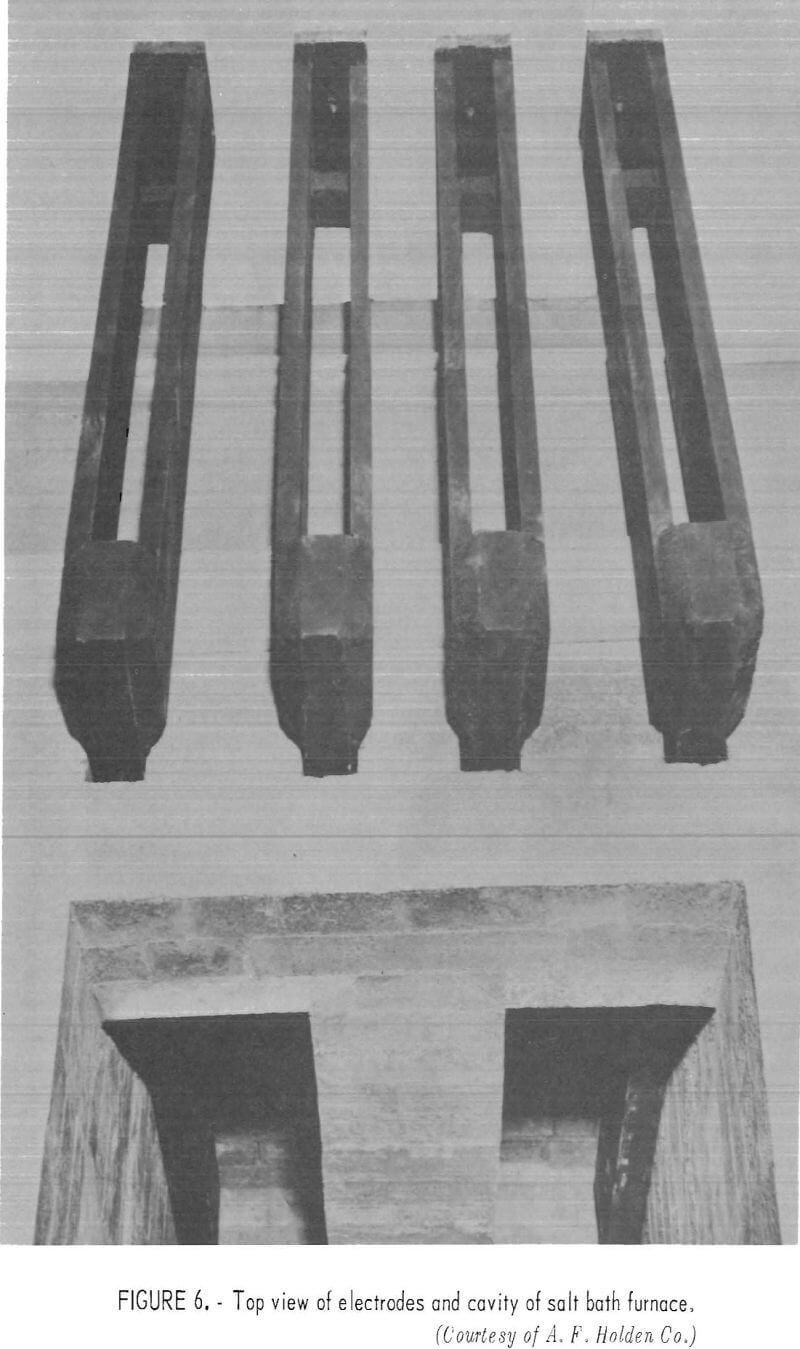
(figs. 5-7), are used for heat treatment of high-speed steel articles and are capable of continuous service at temperatures up to 1,300° C.
Cost Estimate
The objectives of the cost estimate are (1) to compare the merits of barium chloride and calcium chloride as neutral baths for sweating copper from starter and generator armatures and (2) to assess the value of coating the armatures by dipping in sodium sulfate and sodium silicate solutions prior to sweating. Estimated costs will also provide a basis for comparing this process with other methods for separating copper and steel in scrap materials
To provide information as to the distribution of costs, the processes were divided into the unit operations of dismantling, dipping, and sweating. Costs were estimated on the basis of a very small operation because of the limited number of starters and generators available at any one location. Owing to the small size and the fact that the sweating plant would probably be added to an existing scrap yard, special conditions could easily exist that would change the estimated costs considerably. However, comparison between different versions of the process should be valid.
Description of Processes
Costs are estimated for six processes, of which three employ a molten barium chloride bath for sweating the copper and three employ molten calcium chloride. Each series of three processes includes the following variations:
(1) Pretreatment of the armatures by dipping in sodium sulfate, (2) pre-treatment in sodium silicate solution, and (3) no pretreatment. The flowsheets for these processes are shown in figures 8-9.
Prior to sweating, both starters and generators must be partially dismantled to expose the copper parts. First, the end plates are removed by loosening the bolts with a speed wrench. This releases the armatures for removal; however, about half the starters have cast-iron solenoid linkage housings that must be broken with a hammer to release the armatures. In addition, about three-fourths of the starter armatures must have one end of the shaft cut off to remove the bendix spring and end plate. This is most efficiently done with a bandsaw. The starters with solenoid linkages also have solenoid cases that must be opened by cutting with an acetylene torch to remove the copper coil. Field coils are broken loose from the stator housing with a power hammer to remove the copper windings. Copper bushings and brushes are readily removed by hand.
In the sweating operation, nine armatures are loaded into a multiple-clamp mechanism, which automatically grips the shaft ends when the mechanism is lifted by a small hoist. While held in this mechanism, the armatures are lowered into an electric, salt-bath furnace containing a 12-inch-square by 24-inch-deep pot of molten barium chloride or calcium chloride at approximately 1,200° C. Tests showed that the copper melts and drops to the bottom of the pot in 3 to 5 minutes; consequently 9 minutes was assumed to be adequate time for charging, sweating, and removing the armatures. After sweating, residual copper and salt is shaken loose in a vibrating screen and returned to the furnace. Salt adhering to the armatures is not recovered.
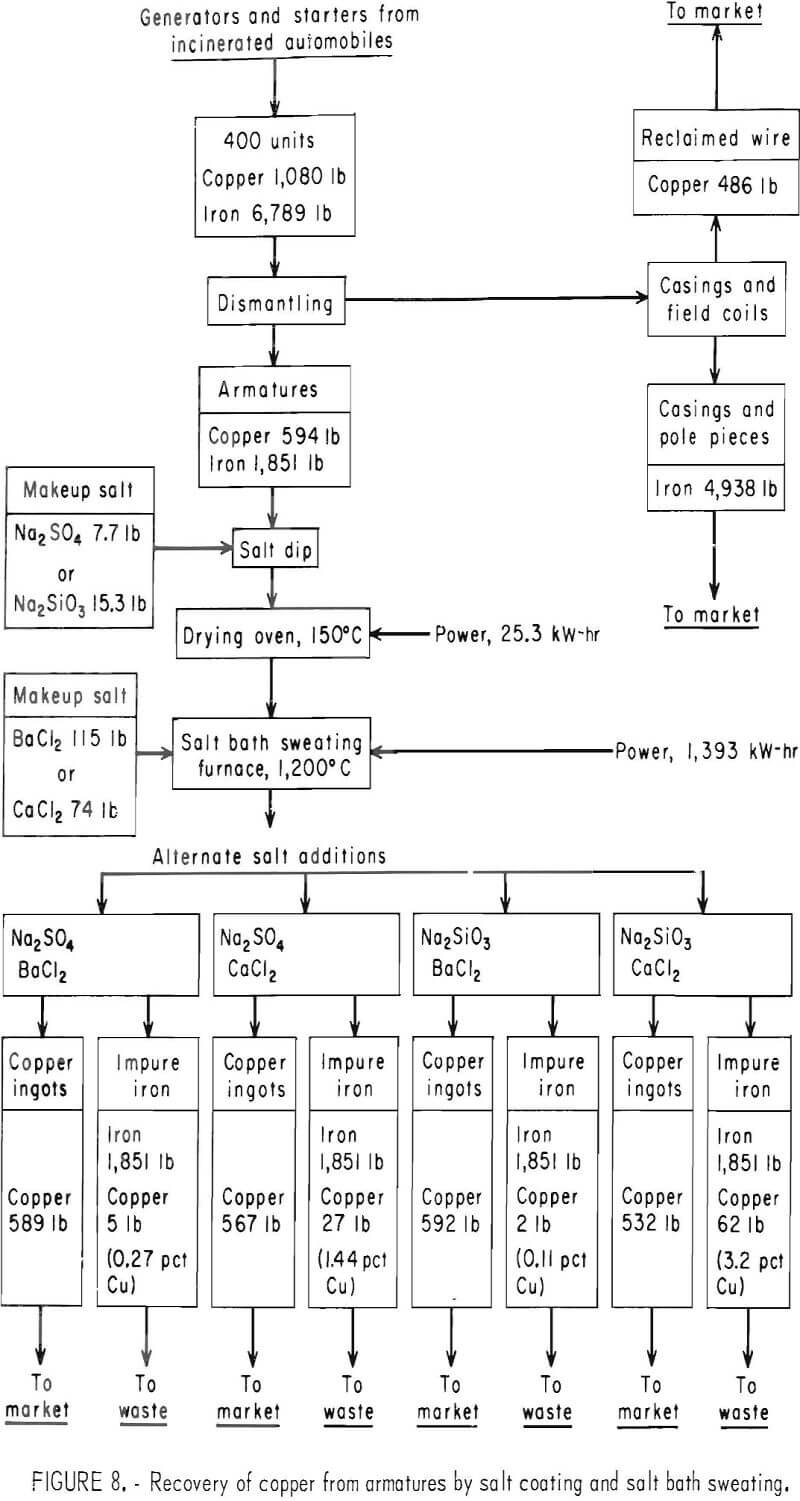
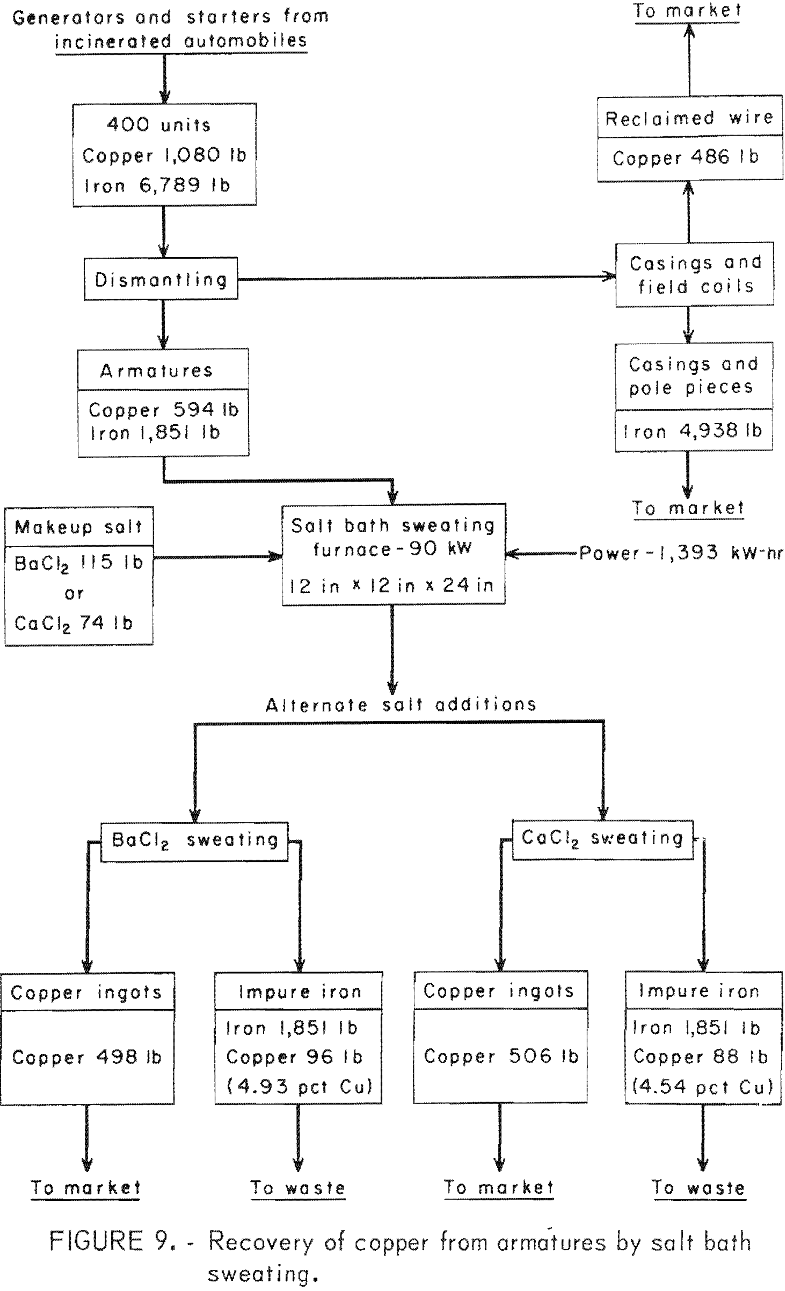
The furnace is large enough to hold a full day’s production of copper. After each day’s operation, the furnace is tilted with a 1-ton hoist, and the salt is decanted into a holding ladle. The copper is then cast into billets for marketing, and the salt is returned to the furnace.
An 8-hour-day, 5-day-per-week operation (250 days per year) was used for this evaluation, and the manpower requirements for the dismantling steps were based on time and motion studies conducted at Salt Lake City. Each of the six processes evaluated require three men for dismantling the starters and generators and another two men for sweating and screening. The four processes in which the armatures are pretreated by dipping in solutions of sodium sulfate or sodium silicate require an additional man.
A byproduct credit of $25 per ton is taken for the iron cases that were assumed marketable as No. 2 heavy melting scrap. No credit is taken for the copper-contaminated armatures. However, armatures dipped in sodium silicate or sodium sulfate and sweated in barium chloride would be salable and would decrease the selling price of copper required for a given return on investment by $0.02 per pound. The copper product includes manually removed copper together with sweated copper.
Estimating Method
The method used for estimating is intended for use with complete chemical or metallurgical plants. However, the results obtained appear to be reasonable based on experience with small-scale construction in such an environment. The scale of this plant is small, and the usual roundoff of costs to the nearest $100 for unit costs and the nearest $1,000 for total costs would have introduced some apparent discrepancies, These discrepancies were avoided by printing all costs to the nearest dollar. Because this was done only for convenience, it should not be taken to indicate a high degree of accuracy.
Accuracy of estimated capital costs should be within ±30 percent.
Capital Costs
Fixed capital costs are based on the delivered cost of individual items of equipment. These costs are for 1971 and correspond to an M&S average equipment cost index of 321.3.
The total direct construction cost for each unit operation consists of the delivered cost of all equipment plus the cost of labor to erect it and the cost of additional construction required to support, house, protect, and make it operable. These various construction costs were estimated by factors applied to the total delivered cost of equipment to be installed. A miscellaneous cost item was added to cover minor equipment or construction that might be overlooked. Equipment not requiring installation, which in this case is a forklift truck, becomes part of the direct construction cost without installation cost or the application of construction factors. This forklift is used for only 1 hour per day; the balance of the time it would be used in the scrap yard. Therefore, only one-eighth the cost of the forklift is charged to these processes.
Forty percent of the total direct construction cost was added as indirect cost, contingency, and fee. This approximates the addition of 10 percent for field indirect expense plus the following percentages of successive resulting subtotals: 5 for engineering plus 5 for administration and overhead, 10 for contingencies, and 5 for contractor’s fees. The field indirect cost covers field supervision, inspection, temporary construction, equipment rental, and payroll overhead.
The fixed capital cost of facilities was estimated as 10 percent of the total fixed capital cost of the production units. These facilities include buildings and equipment for offices, shops, and warehouses, plus fencing, roads, fire protection facilities, and safety equipment.
Utilities were estimated as 12 percent of the total fixed capital cost of the production units. This cost includes the cost of construction and equipment necessary to conduct the utilities to the plant from an outside source and to distribute them to the various operating units of the plant.
The working capital requirements were estimated as the sum of 1 month’s supply of raw materials, 1 month’s out-of-pocket expense (operating cost less depreciation), and 2 month’s product inventory.
Operating Costs
The cost of raw materials includes the shipping cost. Electricity was the only utility used. This was estimated at a rate of 15 cents per kilowatt-hour because of low consumption, Direct labor was based on a rate of $4.00 per hour, and the cost of supervision was taken as 15 percent of this labor cost. Plant maintenance labor varies between 2 and 3 percent of the fixed capital cost without interest for the various operations. Maintenance labor supervision is 20 percent of the labor cost. Maintenance material was assumed to cost the same as maintenance labor. Payroll overhead was estimated as 25 percent of the cost of labor and supervision for operation and maintenance. Operating supplies were assumed equal to 20 percent of the cost of maintenance. These costs constitute the direct operating cost of the plant.
Indirect operating costs include the cost of maintaining the plant facilities and utilities, the expense of accounting, engineering, plant protection and safety, warehousing, shipping, and offices. The indirect cost was estimated as 40 percent of the cost of labor, maintenance, and operating supplies.
Fixed costs include the annual cost of taxes, insurance, and depreciation of the plant. Taxes and insurance combined were estimated as 2 percent of the fixed capital cost including utilities and facilities without interest. Depreciation cost was estimated on the basis of straight-line depreciation over a life of 12.5 years (8 percent per year).
Evaluation, Results, Discussion, and Conclusions
The six processes evaluated were developed to treat the limited number of automobile generators and starters that might be available at a large scrap processing yard; therefore, the scale of operations had to be small. Although it would appear that a more profitable operation could be obtained by increasing the size of the plant, the purchase and shipping costs required to bring raw materials from other locations could offset any gains.
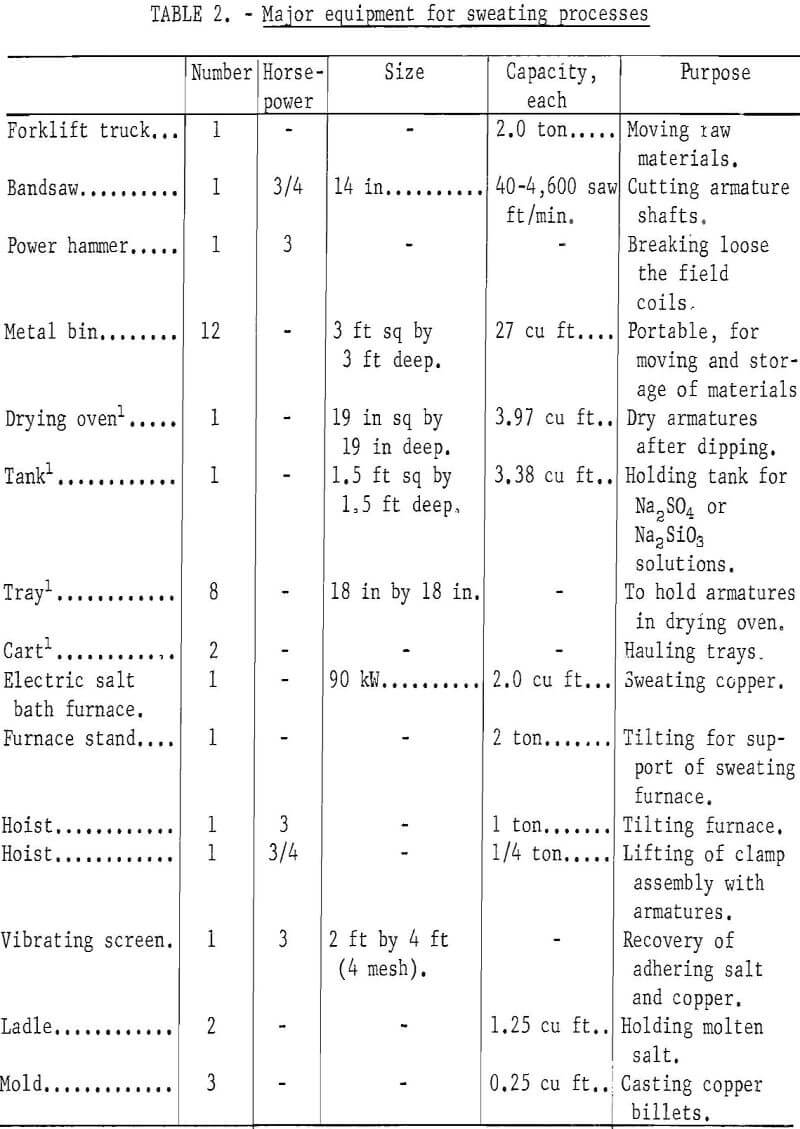
Major items of equipment for these processes are shown in table 2. Fixed capital cost of equipment is the same for the unit operations required by each of the processes evaluated. Examples of equipment costs for the dismantling, dipping and drying, and sweating operations in the process using Na2SO4 coating and BaCl2 drying are presented in appendix tables A-9 , A-10, and A-11, respectively.
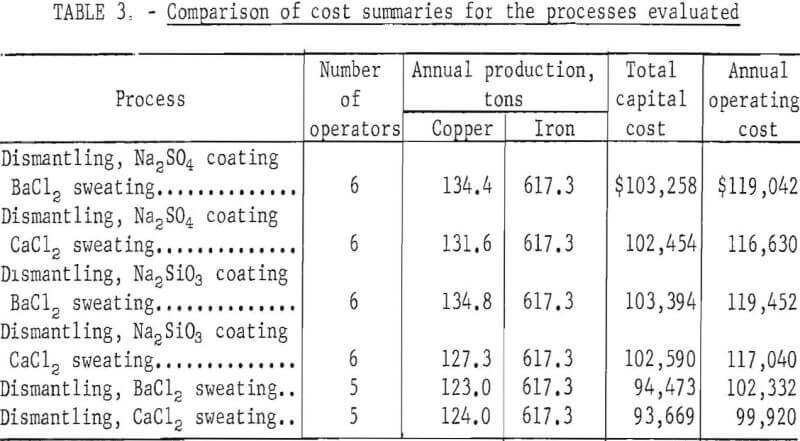
Comparisons of the processes showing manpower requirements, the annual production of copper and iron, total capital cost, and the annual operating cost are shown in table 3.
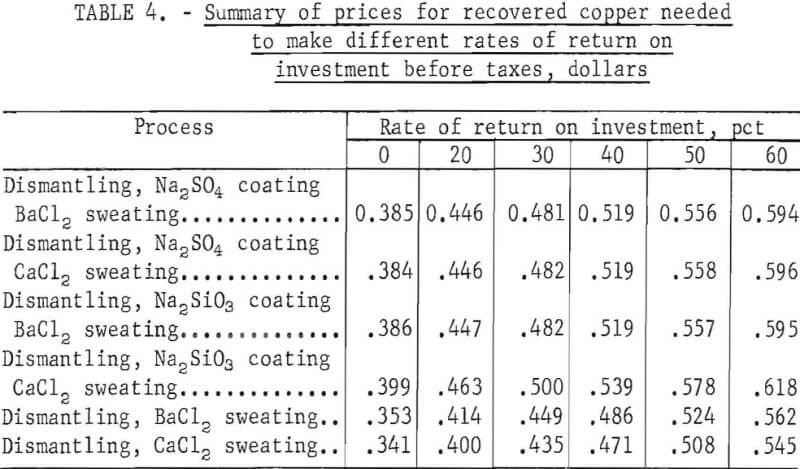
Other comparisons are made in table 4 that show copper product prices for assumed rates of return on investment, of 0 through 60 percent before taxes. These comparisons show that copper product prices required for different rates of return on investment for the six processes have too little variation to conclusively indicate which is the most economical.
Note– These data were extracted from tables A-18 through A-23 in the appendix.
Note.-These data were extracted from tables A-18 through A-23 in the appendix
The four processes using pretreatment (salt dip) give a higher yield of copper product than do the two processes not using a pretreatment, and of these four processes, the two using a barium chloride bath yield the highest copper recovery. However, the two processes not using a pretreatment and recovering less copper require a lower capital investment and one less operator. Economic data for these two processes, which are nearly identical, indicate that the lower operating cost tends to compensate for lower copper recovery, and the return on investment is about the same as the other processes. The simplicity of the processes without a pretreatment and their lower manpower requirements would probably make them the most favorable for scrap processors.
A detailed breakdown of operating costs for the six processes evaluated in appendix tables A-12 through A-17 shows that for the four using a pretreatment, slight variations were caused by minor differences in the cost of Na2SO4, Na2SiO3, BaCl2, and CaCl2. Operating costs for the two processes not using a pretreatment were considerably lower, primarily because one less operator was needed and also, to a lesser extent, because the salt dip was eliminated.
The aforementioned operating costs were calculated on the assumption that these plants would be part of a scrap yard and that the starters and generators would be available at negligible cost. These units were, therefore, listed as a raw material with $0.00 cost. However, if they had to be purchased, their cost delivered would be between $20 and $60 per ton. The effect that the cost of generators and starters has on a process can be shown by its effect on the selling price of recovered copper to obtain a given rate of return on investment before taxes. The effect was evaluated over a range of 0 to 60 percent for each of these six processes, and the data were similar, A typical example of this effect for Na2SO4 dipping and BaCl2 sweating is plotted in figure 10. This plot shows that if the copper product can be sold for 50 cents per pound the return on investment before taxes would not be more than 35 percent if $0 per ton were assigned to the cost of generators and starters. Also, in order to obtain a return on investment of 20 percent, the starters and generators would have to be purchased at about $15 per ton. This would limit the use of these processes to scrap yards that recover their own starters and generators.
The application of this process to automobile generators and starters tends to hide the favorable features of copper removal by sweating because about half the operating costs are in dismantling. Data from the sweating operation alone can be used as a basis for estimating the treatment cost of other copper-containing materials that do not require as much preliminary disassembly as starters and generators.

