Table of Contents
Several hydrometallurgical leach processes have been recently proposed for the recovery of copper from copper concentrates. These include the Cymet Process of Cypress Metallurgical Processes Corporation, the Arbiter Process of the Anaconda Company, and the Treadwell Process of Treadwell Engineering Company. These processes and similar processes for recovery of other minerals, have several phenomenological similarities which make their quantitative description difficult at best.
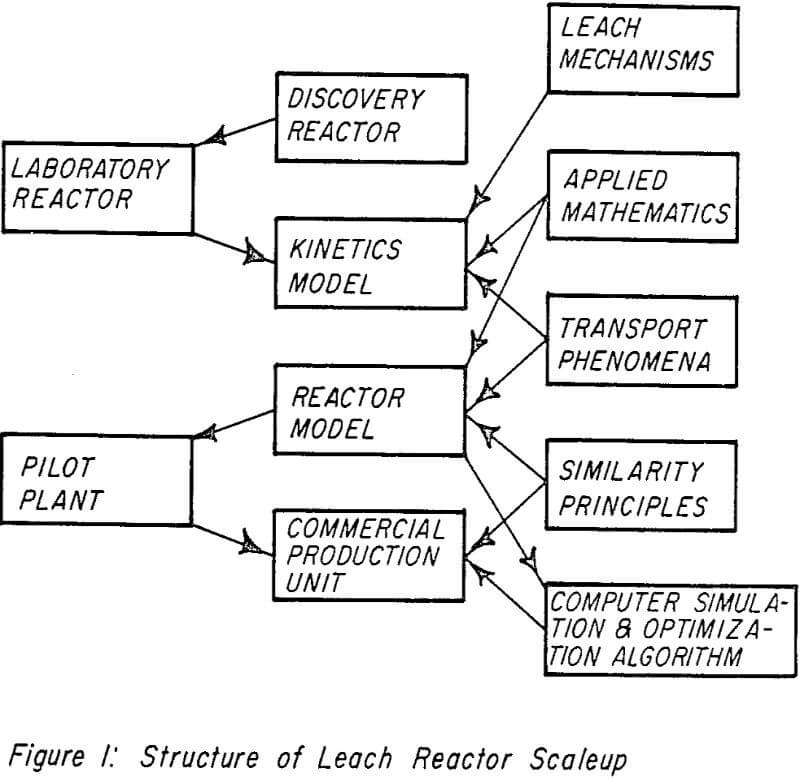
Kinetics Model
The kinetics model is based upon some stated leach mechanism. To model this process mathematically, a few assumptions are necessary. For example, we can assume that all the resistance to mass transport of chemical species is lumped into a “film” surrounding the concentrate particle and that the leaching agent reacts without adsorbing on the solid surface. With these assumptions, mathematical relationships can be written describing the rate at which each step proceeds.

In these equations, the r represents elementary step rates, kLa is the product of the mass transfer coefficient and the solid-liquid interfacial area, kr is the surface reaction rate coefficient, n is the order of the chemical reaction, CAB and CMB are the concentrations of A and M in the bulk liquid phase, and CAi and CMi are their concentrations at the solid-liquid interface. The mineral dissolution reaction has been assumed irreversible which eliminates the need to incorporate the rate expression for dissolved M (Equation 1c).
Reactor Model
In describing the mixing that occurs within a chemical reactor, two idealized cases are often used. These are total backmixing, for which the reactor contents may be assumed to be microscopically uniform throughout; and plug flow, for which no backmixing occurs. In reality, particularly when multiple phases are involved, reactor behavior is intermediate to that predicted by these extremes. Of the many kinetic studies which have been conducted in the area of mineral leaching, almost invariably, steps are taken to insure near total backmixing. This is relatively easy to do in small laboratory reactors, and considerably simplifies the analysis of data. It also results in minimizing or altogether eliminating mass transfer resistances in the leaching mechanism. This is fine so long as the experimental results are to be applied to a case where surface kinetics are rate controlling. Unfortunately, this may not be the case in the larger pilot plant and production reactors. What is necessary then is to find, in addition to the purely chemical kinetic steps, the relationships of the diffusional and mixing phenomena to the process variables and reactor geometry.
This may be accomplished by operating the laboratory reactor under agitation conditions which make its diffusional and mixing behavior analogous to a “similar” reactor on a larger scale. The concept of similarity will be dealt with in the next section. For purposes of illustration, assume that such a “detuned” reactor has a two cell flow pattern (determined by residence time distribution measurements).