Table of Contents
- Roasting Ferruginous Zinc Sulfide Ores
- Experimental Electric Roasting Furnace
- Analytical Methods
- Results of Experimental Roasting in Electrically Heated Roaster
- Roasting in a Hand-Rabbled Reverberatory Furnace
- Roasting in Wedge Furnaces
- Wedge Furnace Roasting
- Roasting
- Roasting of Zinc Sulfide to Oxide & Sulfate
- Formation of Zinc Ferrites
- Solubility of Iron in Roasted Calcines
- Gas Volumes, Gas Temperatures, Stack Heat Losses
Roasting Ferruginous Zinc Sulfide Ores
Mr. J. B. Keating was developing an electrolytic-zinc process for application to the ores of the Bully Hill mines of the General Electric Co. These ores consist of blende and pyrite so finely crystallized and so intimately mixed that no mechanical separation of the individual minerals had been found possible. The ore was reduced to about 60 mesh and roasted in a hand-rabbled reverberatory furnace preparatory to leaching with sulfuric acid. The extractions obtained were quite irregular, varying from a minimum of about 70 per cent, to a maximum of about 90 per cent. This irregularity led to the construction of a small electrically heated roasting furnace, and to the study of roasting under definitely controllable conditions. From time to time, these studies have been extended to other ores. Several commercial-plant operators profess to have gained useful information from the results of these studies and it is hoped that their usefulness may be extended by this publication.
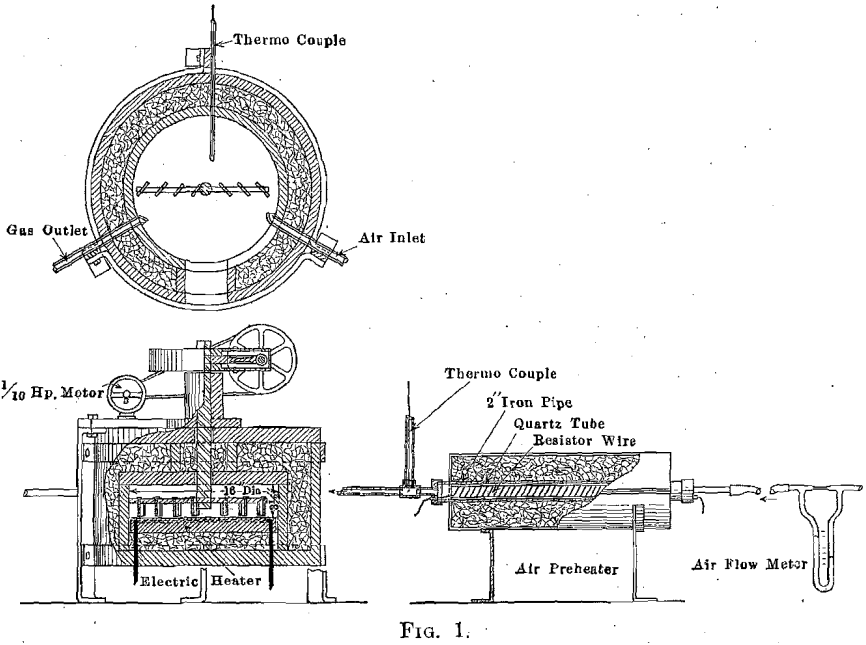
Experimental Electric Roasting Furnace
The furnace used for experimental work is shown in Fig. 1. One fireclay sagger, or pot, was set within another and the space between the two filled with Silox heat insulation. The hearth is a cast-iron plate with an imbedded ribbon of nichrome wire; this wire heater is connected to a potential regulator, which permits a very close voltage control. A mechanically driven arm is fitted with rabble blades so arranged that a uniformly thick ore bed may be maintained indefinitely. This arm is usually driven at about one revolution per minute but its speed of rotation can be varied as desired. Compressed air is led into the furnace through a meter, and it was found necessary to preheat the air in order to secure the definitely isothermal conditions sought.
With this arrangement, it is possible to maintain any desired temperature constant within about 5° C. for any desired length of time and to maintain temperature alike in the hearth, in the ore bed, and in the space above the ore bed. Heraous (Pt-Pt-Rh) thermocouples were kept in the hearth, in the space above the ore bed, and in the air feed-pipe and were connected to recording instruments. The couples were frequently calibrated, and considerable pretense to accuracy is claimed for the temperatures recorded in connection with this work. Gas samples were taken at the air outlet of the furnace, and calcine samples were taken at frequent intervals.
Analytical Methods
Sulfur-dioxide concentrations in the roaster gas were determined by absorption in standard iodine solution and titration with thiosulfate. Water-soluble zinc in the calcines (zinc sulfate) was determined by bringing to a boil 5 gm. samples of calcine together with 200 c.c. of water, and by titration of filtered off solution with ferrocyanide. Total soluble zinc was determined by boiling, for 6 min., 1 gm. samples of pulverized calcines together with 0.75 gm. sulfuric acid and 100 c.c. water. (This gives a solution only faintly acid; the test is wholly an arbitrary one that has been found to give consistent results.) The filtered solution is titrated with ferrocyanide after the usual fashion.
Insoluble zinc is determined in the residuum from the soluble zinc determination, and the extractions reported refer to soluble zinc/soluble zinc plus insoluble zinc.
While this method of determining extraction is more tedious than one involving the determination of the total zinc directly, it leads to rather more consistent results and also to the direct determination of the insoluble zinc, which, after all, is the most important of determinations.
Sources and Nature of Ores Tested
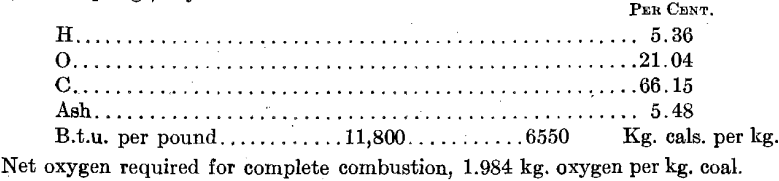
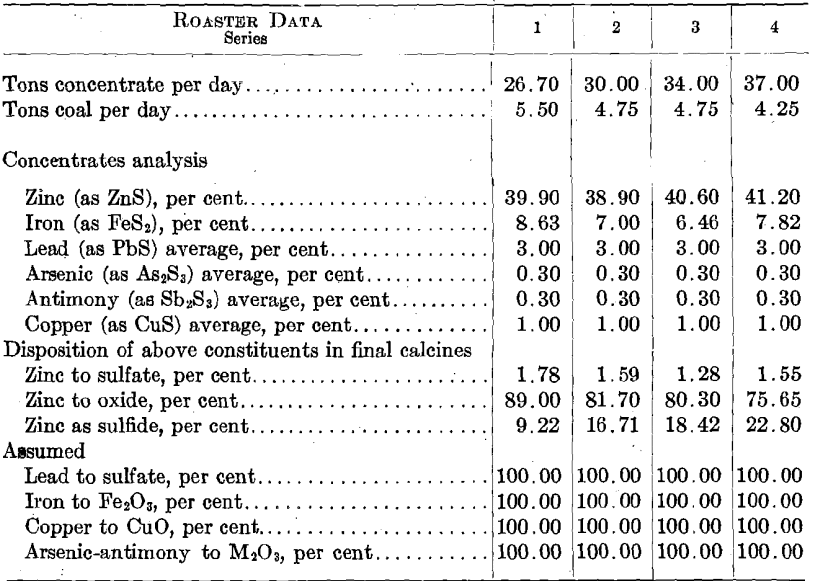
Bully Hill, Bully Hill Copper Mining Co,, Shasta Co., Calif., is an unconcentrated ore, massive sulfide. The minerals are apparently sphalerite and pyrite and are so finely crystallized, and finely intermixed as practically to defy separation. The gangue material is mainly barium sulfate. Butte Superior, Butte Superior Copper Mining Co., Butte, Mont., flotation concentrates obtained in 1912 were apparently a mixture of pure sphalerite and pyrite. Daly Judge, Judge Mining & Smelting Co., Park City, Utah, table and flotation concentrates are pure sphalerite and pyrite, quite coarsely crystallized. Individual zinc crystals contain no iron or lead and individual pyrite crystals contain no zinc. Broken Hill, Amalgamated Zinc (DeBavay’s) Ltd., Broken Hill, Australia, flotation concentrates have iron combined with zinc as ZnS:FeS. Frisco, Federal mines, Coeur d’Alenes, Ida., 1916, apparently a flotation concentrate in which iron is apparently combined with zinc as FeS:ZnS.
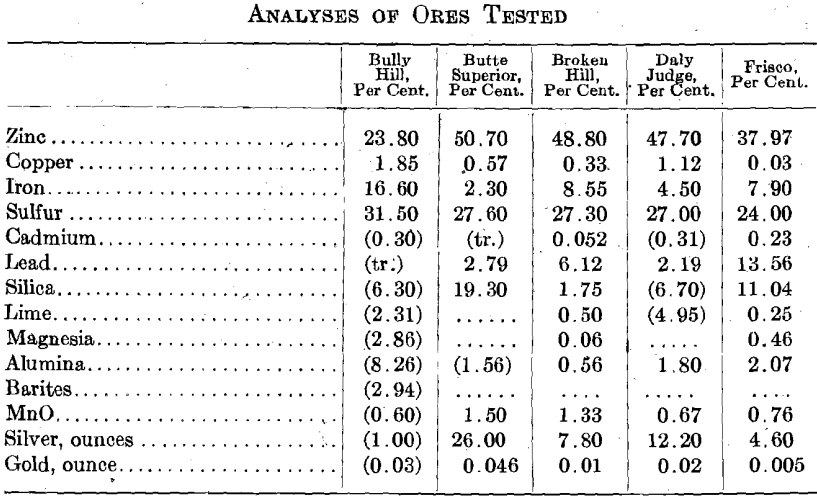
Figures given in parentheses refer to analyses of samples other than those specifically tested.
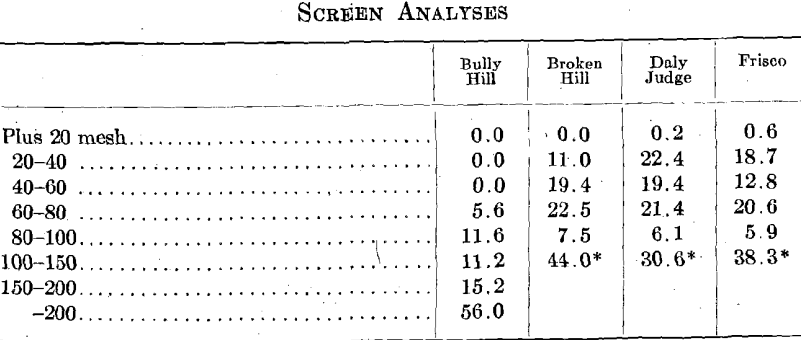
No screen analysis record of the Butte Superior concentrate is at hand. The material was very fine, most of it probably passing through a 100- mesh screen.
Results of Experimental Roasting in Electrically Heated Roaster
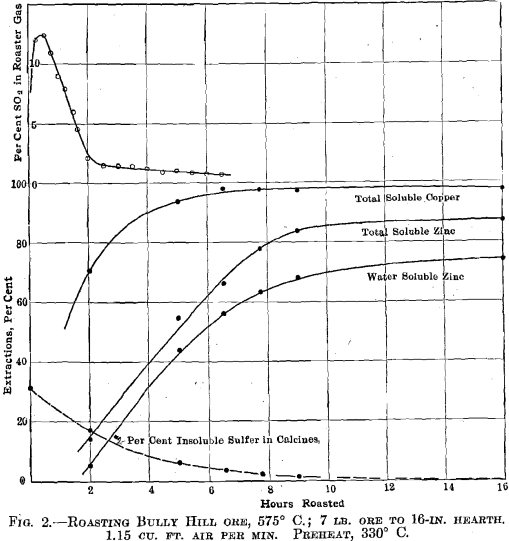
Bully Hill Ore.—This material was roasted isothermally at 425°, 450°, 475° C. and so on at 25° intervals up to the limit of the roaster,
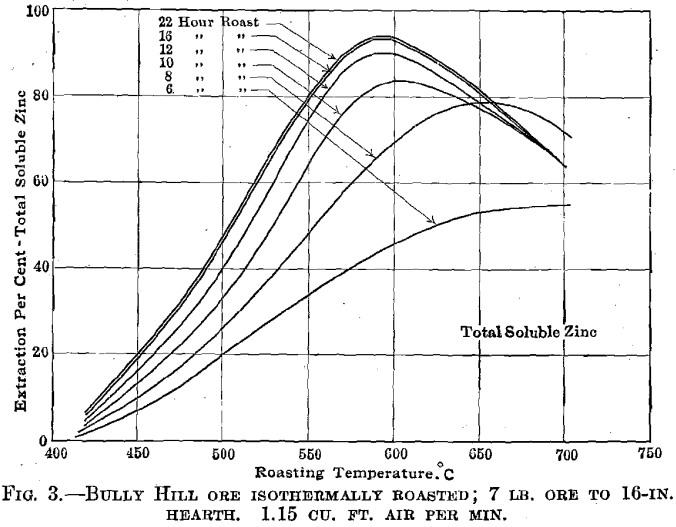
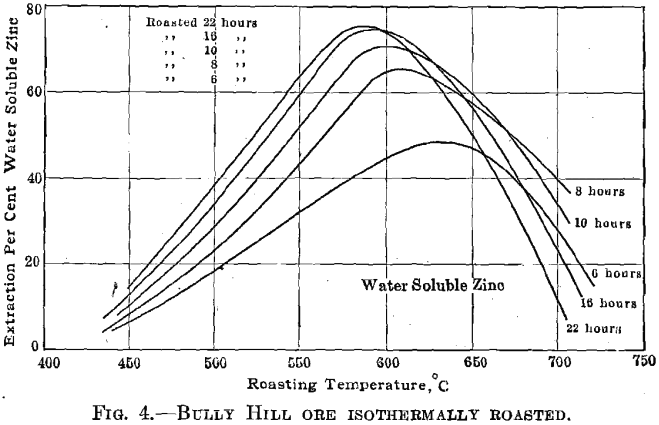
700° C. Fig. 2 shows the results obtained at the optimum temperature 575° C. Similar plots were made for each roasting experiment, and the results, in slightly idealized form, are shown in Fig. 3. The summarized data relative to the formation and decomposition of zinc sulfate are given in Fig. 4.
The sulfur-dioxide concentration curve in Fig. 2 shows quite markedly the flashing off of the pyrite sulfur and that practically all of such sulfur is oxidized before the zinc sulfide is appreciably reacted upon. No water-soluble zinc sulfate is formed until the sulfur-dioxide concentration falls below some 2-3 per cent., but beyond the time at which the sulfur-dioxide concentration reaches this low value practically all of the zinc sulfide oxidized was converted to zinc sulfate. Thus between the second and sixteenth hour, the gain in total soluble zinc was 73 per cent, and the gain in water-soluble zinc was 69 per cent.
The effect of varying the amount of air fed to the roaster is indicated by the following:
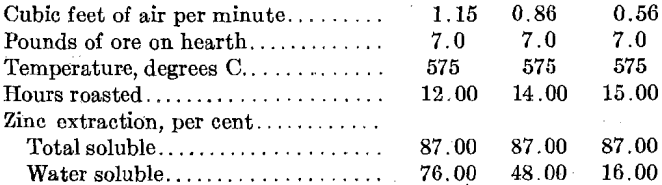
The formation of zinc sulfate is important in so far as water-soluble sulfate in the calcines reduces the amount of acid required from outside
sources for the leaching operations. Ordinarily, if the calcines contain 3 to 4 per cent, of water-soluble zinc, based on the calcines weight, the zinc plant becomes self-supporting in its acid requirements.
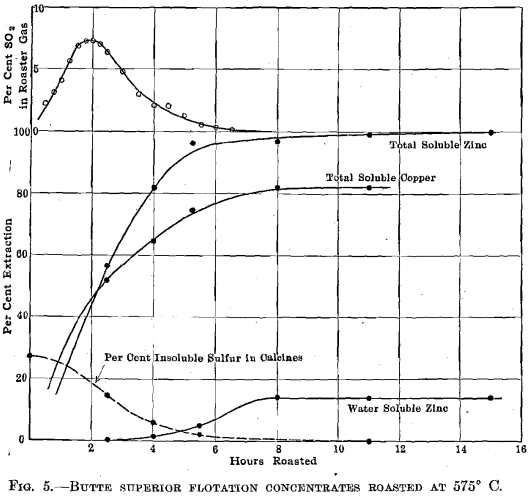
Butte Superior Concentrates.—Fig. 5 shows the behavior of finely divided high-grade zinc concentrates roasted at 575° C. The sulfur
dioxide concentration curve shows no flashing off of large quantities of pyrite sulfur. The oxidation of zinc sulfide begins almost immediately but there is no formation of sulfate until the sulfur-dioxide concentration has fallen to about 2 per cent.; beyond this point there is a gain of 14 per cent, water-soluble zinc, as compared with a gain of 17 per cent, in total soluble zinc.
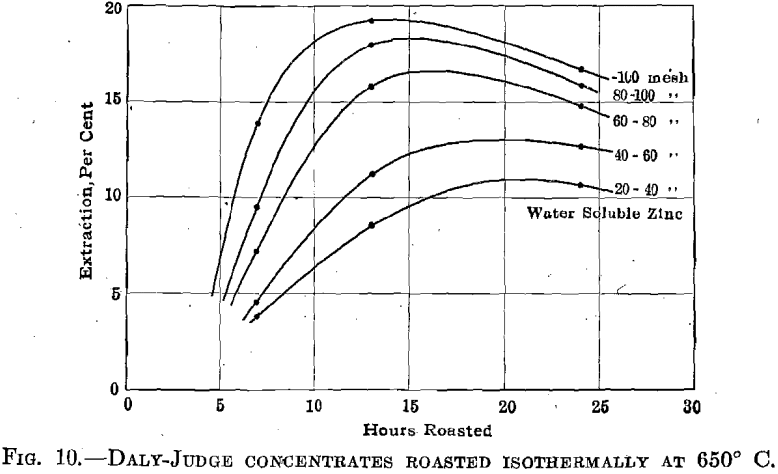
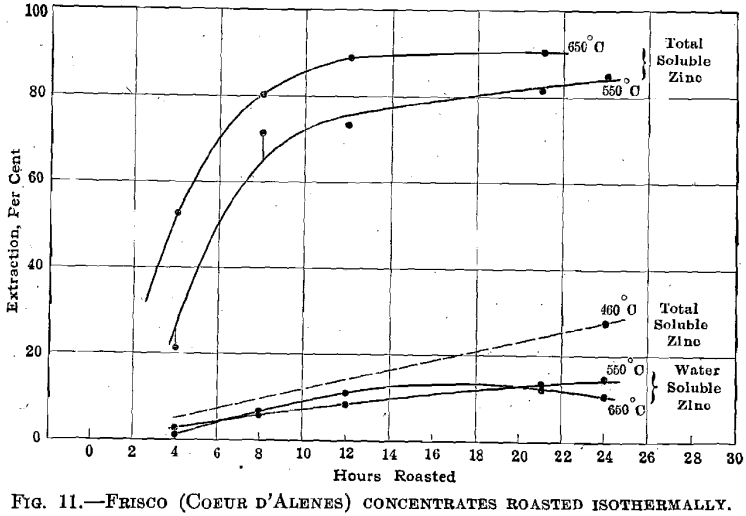
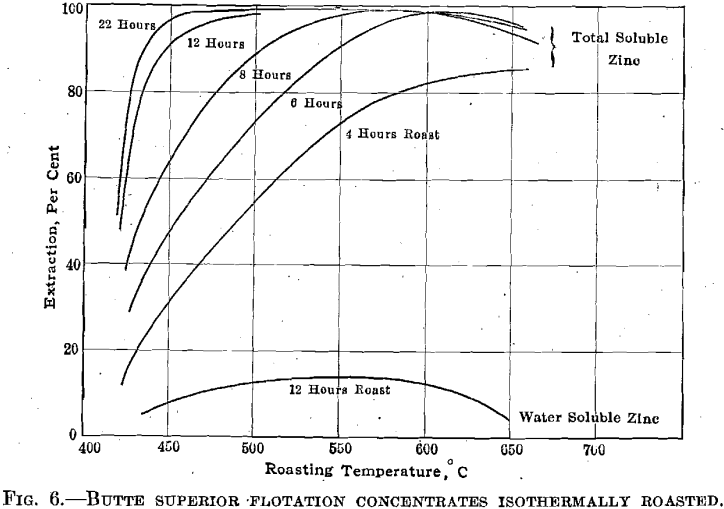
Fig. 6 summarizes very broadly the results obtained during four experimental roasts at 450°, 500°, 575°, and 650° C. It is curious to note that 22 hr. roasting at 450° C. rendered 97.6 per cent, of the zinc soluble. The calcines obtained from the 450° roast accounted for all but 0.75 per cent, of the original silver content. The calcines obtained at 650° C. showed a loss of 13.9 per cent, of the original silver content. The writer understands that the roasting of similar concentrates at Bartlesville led to a silver loss of 26 per cent. The roasting at Bartlesville was accomplished in Zellweger furnaces where the concentrates were exposed 24 hr. to temperatures above 900° C. No pretense is made that the above silver determinations are more than merely indicative of the relations between silver loss and roasting temperature.
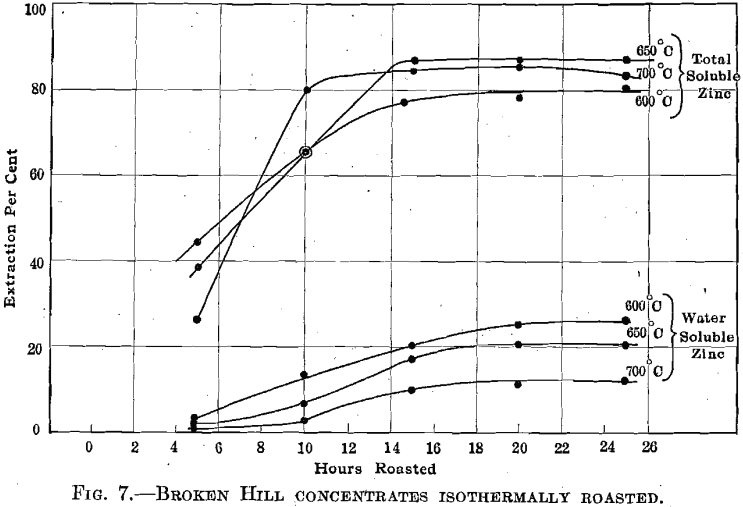
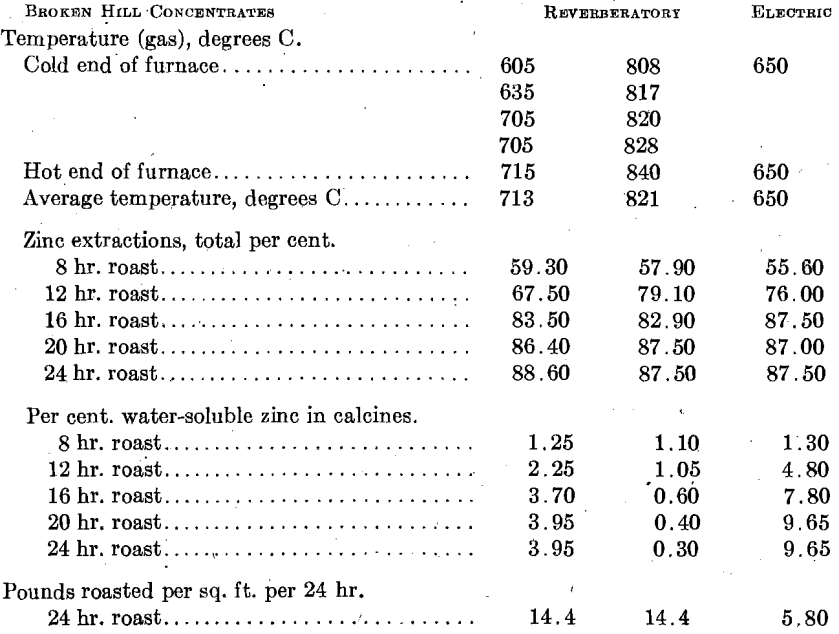
Broken Hill Concentrates.—Fig. 7 summarizes the results obtained in roasting this material at 600°, 650°, and 700° C. It is apparent that the coarser screen size leads to a longer roasting period. In spite of the low iron content of these concentrates, we could not obtain extractions higher than 87 to 88 per cent. Similar concentrates roasted at high temperatures in Matthiessen Hegeler kilns yielded 87 per cent, extractions; roasted in a hand-rabbled reverberatory at high temperatures and at low temperatures also yielded 87 to 88 per cent, extractions. It appears that the zinc is combined with iron in the original mineral and that no manner of straight roasting will serve to break up the combination. This behavior is thoroughly characteristic of several isomorphous zinc- iron minerals (marmatites) with which we have experimented.
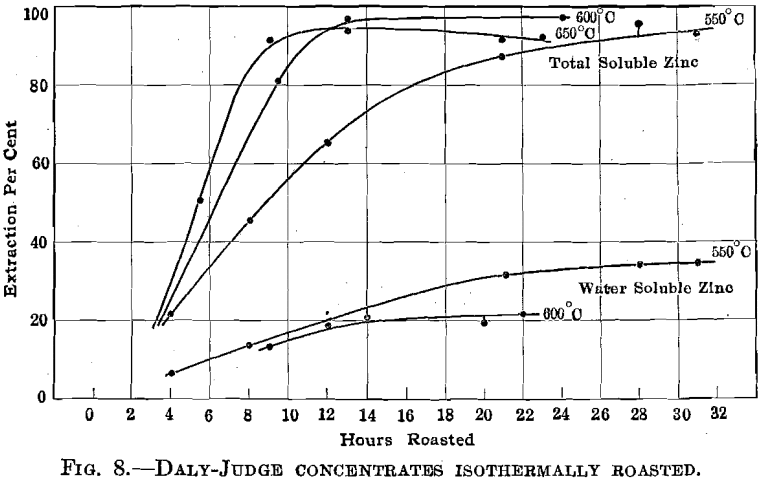
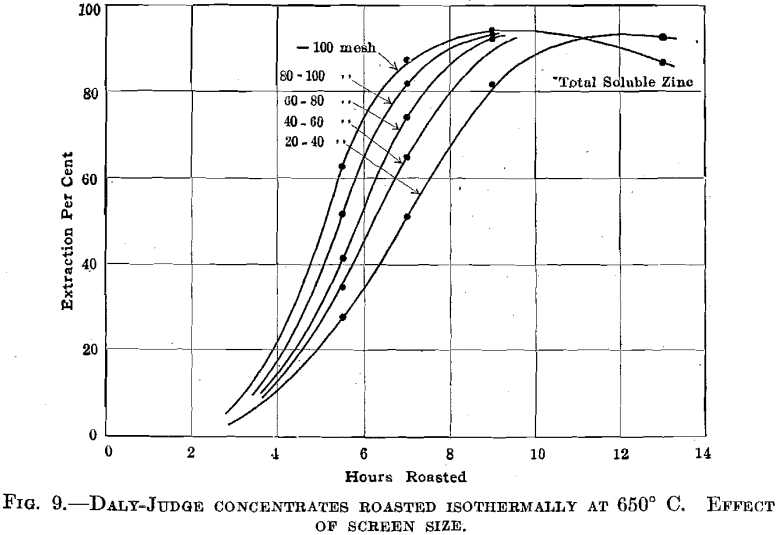
Daly Judge Concentrates.—Fig. 8 summarizes the results obtained in roasting this material at 550°, 600°, and 650° C., while Fig. 9 indicates the effect of screen size upon roasting rates. There is an excellent indication that in roasting a mixture of variously sized ore particles to secure maximum extraction for the mixture the fines will be over roasted. An interesting relation between screen size and the formation of zinc sulfate is brought out in Fig. 10.
Frisco Concentrates.—Fig. 11 summarizes the results obtained in roasting this material at 460°, 550°, and 650° C. This concentrate strongly resembles the Broken Hill concentrate in appearance and in its behavior in the roaster.
Roasting in a Hand-Rabbled Reverberatory Furnace
The reverberatory roaster at Bully Hill had a hearth 8 ft. (2.4 m.) wide by 13 ft. (3.9 m.) long and was divided into two 4-ft. strips by a vertical partition wall. Ore was fed in periodically at one end of the two hearth strips, and periodically moved forward toward the discharge end. At the discharge end was placed a small combustion chamber into which oil was injected with steam. Roasting was carried on continuously and the following data are characteristic of continuous operating conditions.
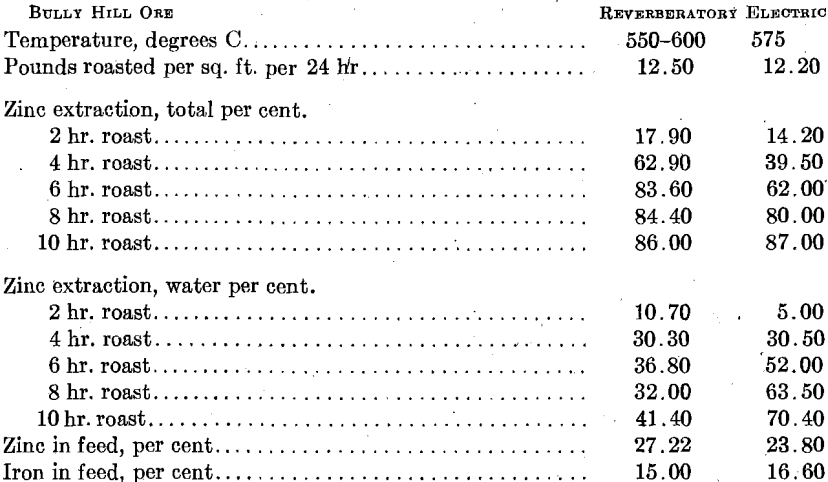
It should be noted that definite time samples are hard to get out of the reverberatory, and that the amount of water-soluble zinc obtained in the electric roaster could be controlled through control of air volume. At the time the above work was done in the reverberatory furnace, every endeavor was made to secure maximum oxidation to sulfate. The comparison then covers maximum possible water-soluble zinc sulfate for the two furnaces.
It is apparent that in these two instances, the only instances where a direct comparison between the electric roaster and the reverberatory is possible from the data at hand, the two furnaces yielded practically identical extractions and that time factors for the two furnaces are at least comparable. The reverberatory furnace was rabbled with hand rakes about as frequently as a man could cover the hearth.
Roasting in Wedge Furnaces
When the first electrolytic-zinc plant, that at Trail, B. C., was built, practically no zinc ores had been roasted in the Wedge type of furnace (including the similar McDougal, Herreschoff, and Skinner designs) so far as the writer can learn. However, a Wedge roaster had been set up and operated in Philadelphia in connection with the manufacture of lithopone, but little information could be obtained relative to its performance. The general practice had apparently developed along the line of long-period roasting at relatively high temperature, Matthiessen- Hegeler, Zellweger, and other types of straight-fine furnace types being used almost exclusively.
The electrolytic-zinc process was developed primarily to treat the western low-grade zinc ores and concentrates. As it is essential, to secure good extraction from low-grade concentrates containing considerable iron, to keep the temperatures in the roaster reasonably low—around 600°. C.—it was thought that the Wedge furnace type could be adapted for this purpose. Since this time, Wedge furnaces have been operated continuously at temperatures approaching 800° C. but this appears to be about the limit, for the maintenance costs become almost prohibitive and frequent service interruptions are caused by the need for repairs. Temperatures considerably above 800° C. are commonly used in roasting for the retort—probably in order to break up the sulfates formed during the earlier stages of the roasting period—and it is probable that the aversion of the retort smelter toward the Wedge furnace type is more or less well founded.
For low-temperature roasting, the Wedge furnace promised economies in lower first cost, lower labor costs, and lower fuel consumptions as compared with the straight-line furnace types, and further incentive to its selection rested in the fact that the Western smelters were most familiar with the type. In any case, each electrolytic plant in the West independently selected the Wedge type of roaster but no published information is available regarding either practice or performance of the roasting equipment at these plants other than the brief statement made by E. H. Hamilton to the effect that the Trail furnaces were treating 50 tons each per day.
It is unfortunate that the entire period of operation of the electrolytic-zinc plants has been overlapped by the war period of abnormal cost and price conditions. Under these conditions, coupled with the fact that a completely new practice had to be developed throughout the zinc plant, the cost of the zinc available in the calcines has not been, by any means, the major item in the cost of producing electrolytic zinc. Almost any sort of roasting practice led to fair recoveries. Improvement in roasting practice could only lead to somewhat better recoveries of zinc and therefore only to the reduction of a minor cost item.
Consequently, less attention has been given the roasting practice than would characterize practice in normal times. This undeveloped state of the roaster practice has unquestionably led to a reluctance to publish accounts of the roasting work already done, a reluctance that is shared by the writer.
All the operating electrolytic-zinc plants are dealing with zinc materials containing important quantities of lead, copper, silver, and gold, which must be recovered by smelting methods from the zinc-plant residues. As the zinc extraction is improved by improvements in roasting practice, the residues will decrease in weight and less penalty will be exacted by the smelter for zinc obtained in them. The savings accruing to a better roaster practice in these respects is quite as important as the direct additional saving of zinc. That which follows in regard to the operation of the Wedge roaster at the Judge zinc plant may properly be considered a mere teething experience but it seems to point the way toward some of
the possible improvements in practice.
Wedge Furnace Roasting
The primary values in the ores of the Judge mines are lead and silver. The ores, however, carry considerable zinc and iron and the company had been marketing a zinc concentrate of the composition indicated in the analyses given earlier in this paper. In order to make a marketable zinc concentrate, considerable zinc was lost in an iron-zinc middling product; much of which was taken over into the zinc concentrate when the electrolytic-zinc plant became available. This led to the zinc plant getting a lower grade of concentrate than that with which the preliminary experiments were conducted, which was compensated for, to a certain extent, by an advantageously high iron content in the zinc-plant residues.
The furnace used for roasting is a standard Wedge zinc roaster, 25 ft. (7.6 m.) outside diameter, 21 ft. (6.4 m.) inside diameter, seven roasting hearths with a total hearth area of 2350 sq. ft. (216 sq. m.). Each hearth is rabbled by two arms, carrying ten rabble blades each. The arms on the upper five hearths are air cooled, 5000 cu. ft. (140 cu. m.) of air being blown through them against a pressure of 2.09 oz. per sq. in. by means
of a No. 9 Buffalo fan blower. The cooling air is discharged into the space above the sixth and seventh roaster hearths. The arms on the lowest two hearths are water cooled, requiring some 20 gal. (75 l.) of water per minute, which later serves as cooling water for the Baker calcines cooler. The central shaft is driven through a Reeves variable-speed drive and its speed of rotation can be varied between the limits 120 and 270 sec. per rev. Two coal-fired combustion chambers are arranged at diametrically opposite points; their flues conduct the combustion gases into the sixth and seventh hearth chambers. Wilson Mauelein pyrometer couples are arranged in grooves cut into the upper surface of each hearth midway of the active hearth radius; all the couples are connected to multiple recording instrument.
The furnace was started with the intention of keeping temperatures as close to 600° C. as possible. It was soon found that with coarse concentrates and a short roasting period, this temperature was too low for any reasonable roaster tonnage capacity. The temperatures were therefore gradually raised to 700° C. and above with the results shown in Table No. 1. The appended comparison between the roasting rates of
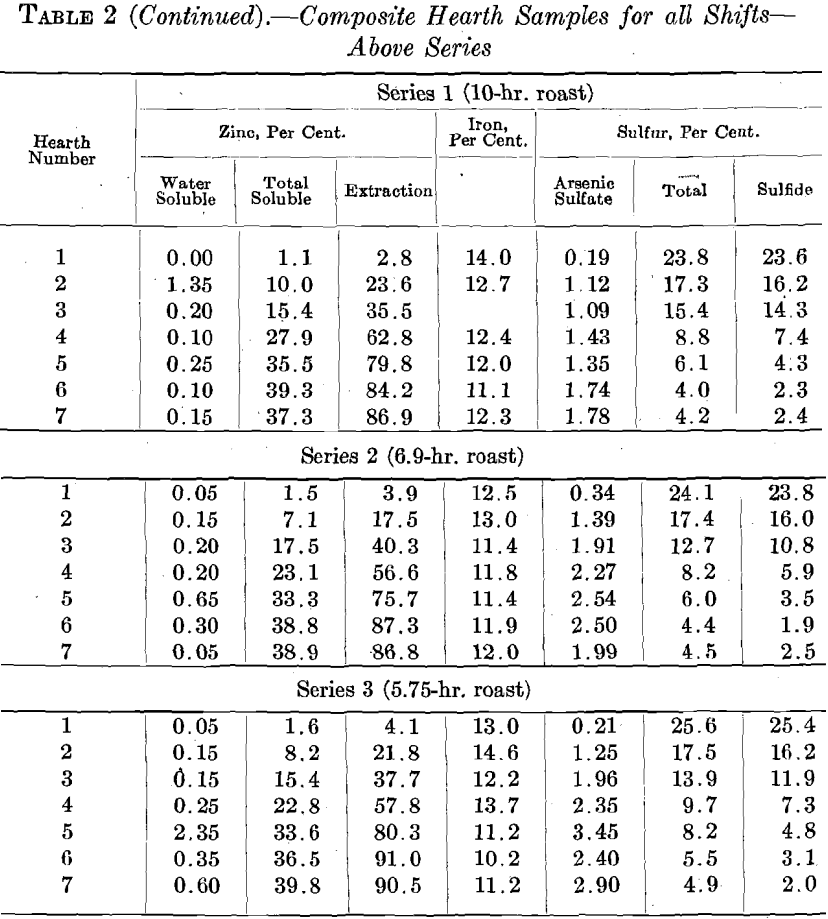
the various screen sizes in the Wedge furnace and in the experimental roaster clearly indicates the need for finer grinding of the roaster feed. Finer grinding was impracticable at the time, as the zinc plant was not equipped for grinding concentrates. It was also known that at Trail the use of a very fine roaster feed led to the collection of 7 per cent, dust in the flues and the Cottrell system installed there; the Judge roaster was not equipped for dust collection. The alternative appeared to be to increase the roasting period by slowing down the speed of rotation of the rabble shaft. The results are shown in Table 2.
The extraction and sulfur elimination data given in Table 2 are shown in Fig. 12. Slowing down the rabble shaft slows down the rabbling rate and also leads to a thicker ore bed upon the hearth. Both of these factors lessen the oxidation rate to such a point as to more than offset the effect of the longer roasting period.
The next set of experiments concerned itself with the relations between the rate of feed to the furnace, extraction, and fuel consumption. Here, again, the effect of screen size upon extraction was noted, with the results given in Fig. 13. Comparing these results with those given in Fig. 9 shows that there is a fairly definitely fixed relation between screen size and roasting rate. There is little question but that the concentrates should be reduced to pass at least a 60-mesh screen. The limiting feature is undoubtedly too low an oxidation rate; i.e., it is failure to oxidize sulfur rather than the formation of insoluble ferrites that leads to low extractions in this case. This is brought out by the data relative to the nature of the insoluble zinc compounds in the calcines, as given in Table 4.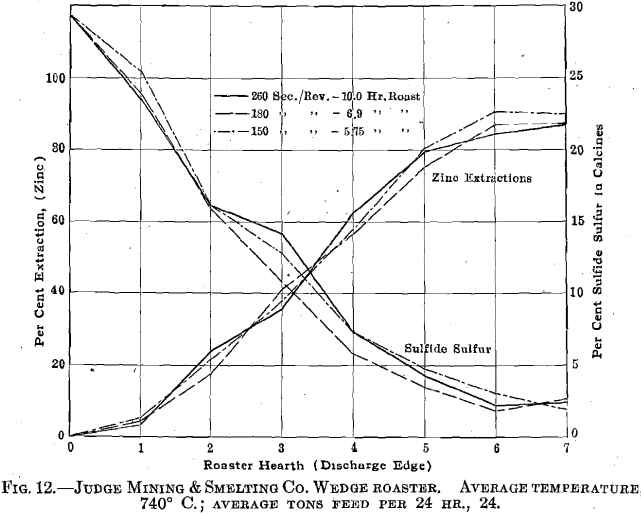
With the furnace draft and temperature remaining as they were during the above experiments and with the concentrates not further reduced in size, the economic capacity of the furnace does not exceed 28 tons per day with a fuel consumption of about 20 per cent, of the concentrates weight. By grinding to 60 mesh, it is probable that the roaster capacity can be increased to rather better than 35 tons per day with a coal consumption not greatly exceeding 10 per cent, of the concentrates weight. It also appeared possible to increase the oxidation rate by increasing the rabbling rate. The number of blades per rabble arm was accordingly increased to 15 and the pitch was proportionately decreased. No detailed experiments have been carried out, however, to determine the difference in furnace behavior. For the month preceding the change,
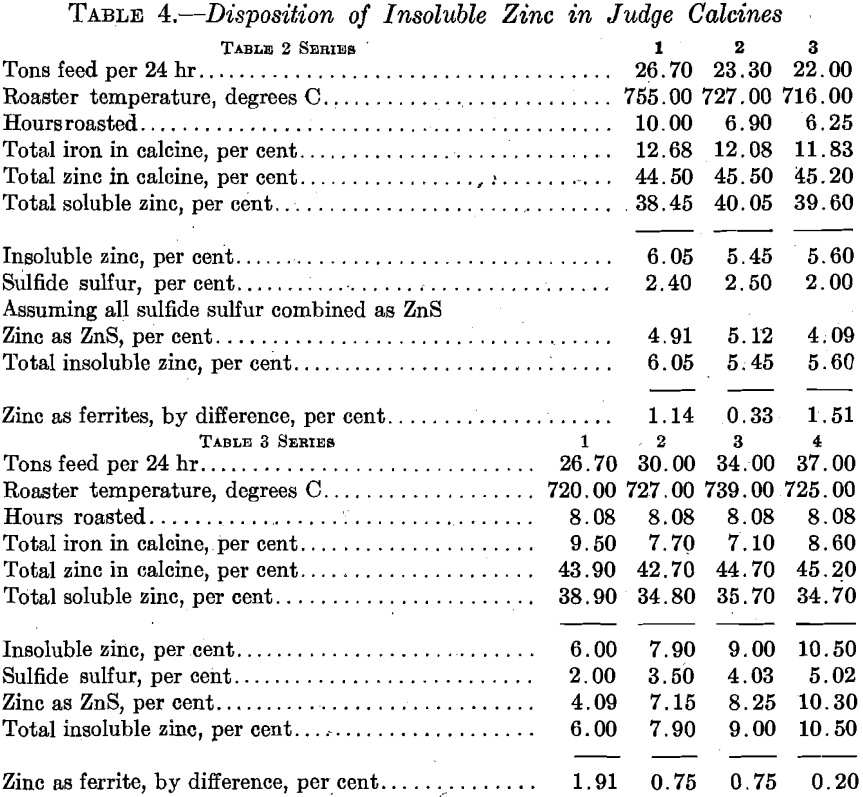
the average tonnage roasted per day was 28, with calcines yielding 90 per cent, zinc extractions. For the month following the change, and with no other alterations in feed or practice generally, the calcines averaged a trifle over 92 per cent, soluble zinc. It appears that at 28 tons feed rate the extraction was improved by about 2 per cent., and that for the same extraction the capacity of the furnace was raised to about 32 tons per day.
A greater rabbling frequency would seem better practice in a new furnace installation, but it would be better to add arms than to add more rabble per arm, as fifteen rabbles per arm brings the blades rather too close together. Reversed blades may be used on one arm on each hearth, as in the small electric roaster, so that the ore bed may be stirred without translating it across the hearth so rapidly.
Greater capacity might be obtained by increasing the roaster temperature, but a material increase in temperature above 750° C. greatly increases maintenance costs and service interruptions.
Roasting
Roasting in General.—Roasting is essentially a diffusion process. Mobile, gaseous oxygen must be brought into contact with relatively immobile, solid, sulfide molecules in order to effect oxidation of the latter. A cube of blende that will pass a 100-mesh screen contains some 10 16 molecules of zinc sulfide of which less than one millionth are freely exposed to oxidation at the surface of the cube. In order to contact with arid oxidize the interior molecules that make up the mass of the ore particle, oxygen must diffuse into the ore particle. It would appear that this alone might fix the relative roasting rates of variously sized ore particles in that given roasting conditions would lead to the oxidation of a surface layer of uniform thickness.
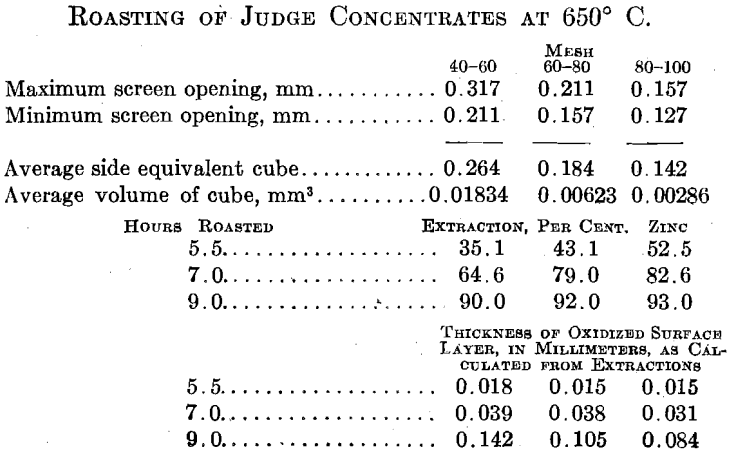
The agreement here is too good to be wholly accidental. The diffusion rate of oxygen into the ore particle, and thus the roasting rate, must be determined by the oxygen concentration at the surface of the individual ore particle. Oxygen must diffuse into the ore bed before it can diffuse into the ore particle itself, and the relative rates of oxidation on the hearth and during the dropping of the ore particles from hearth to hearth through the roaster gas and the relative rates of oxidation in the ordinary roaster, as compared with the Dwight Lloyd machine or the “porous hearth” blast roaster described by Greenawalt, would indicate that the first diffusion step is as great a limiting feature as is the second.
Diffusion into the ore bed can be, in part, set aside by mechanical means, that is, by rapid and frequent stirring or rabbling, or by forcing the roaster gas through the ore bed. For example, it is reported that considerable success has been attained in Australia recently in blast roasting zinc-sulfide ores for the retort smelter. Diffusion into the ore particle itself is beyond control except so far as it can be accelerated by increasing temperatures. It will reach its maximum rate when the oxygen concentration at the surface of the ore particle is the same as the oxygen concentration in the roaster gas as a whole. This is very important in its bearing upon fuel consumption in roasting. The energy of combustion of the average zinc concentrate is sufficient to heat to the roaster temperature some three times the amount of air required for its oxidation yet the cost of the fuel used for roasting zinc concentrates is by far the greater item in the cost of roasting. This will be taken up later in discussing the energy balance determined for the Wedge roaster at the Judge plant.
Roasting of Zinc Sulfide to Oxide & Sulfate
Zinc sulfate can be formed through any one of the following reactions:
ZnS + 4O = ZnSO4
ZnS + 3O = ZnO + SO2
ZnO + SO2 + O = ZnSO4…………………………..(a)
ZnO + SO2 + Fe2O3 = ZnSO4 + 2FeO………………..(b)
2FeO + O = Fe2O3
ZnO + SO3 — ZnSO4………………………….(c)
There is considerable evidence that the first reaction is responsible for most of the sulfate formed. The only gaseous reagent is oxygen and there are no gaseous reaction products, therefore, the oxygen concentration alone should mainly determine the amount of sulfate formed. Reactions (a) and (b) involve two gaseous reagents, so that the amount of sulfate formed will be determined mainly by the product of the concentrations of oxygen and sulfur dioxide.
In the case of Bully Hill ore, see Fig. 2, no appreciable amount of sulfate was formed until the sulfur-dioxide concentration fell below 2 per cent. Beyond this point, when the sulfur-dioxide concentration averaged much below 1 per cent., 73 per cent, of the zinc was oxidized and 94.5 per cent, of that was oxidized to sulfate. Of the added sulfur oxidized during this later stage, more than 50 per cent, was converted to sulfate. It was found that increasing the air rate, which raises the oxygen concentration in the roaster gas and lowers the sulfur-dioxide concentration, rapidly increased the amount of sulfate formed.
In roasting the high-grade Butte Superior concentrates, see Fig. 5, no appreciable amount of sulfate was formed until the sulfur-dioxide concentration fell below 2 per cent. During the first 4 hr. of the roasting period, when the sulfur-dioxide concentration exceeded 2 per cent., 82 per cent, of the zinc was oxidized. During the later period, and while the sulfur-dioxide concentration averaged so low that one could breathe the roaster gas without serious discomfort, an added 17 per cent, of the zinc was oxidized and 85 per cent, of this was converted to sulfate. Finely ground ore should lead to higher oxygen concentrations at the contact between the interior zinc-sulfide molecules and the roaster gas, since it should lead to thinner diffusion diaphragms; it is to be noted that fine ore particles consistently lead to greater amounts of zinc sulfate.
It is by no means clear why a high iron content should lead to the formation of more sulfate than a low iron content, but when the low iron content Butte Superior concentrate was roasted in the same manner as the high iron Bully Hill ore, the Butte Superior concentrate yielded only 20 per cent, of its zinc as sulfate while the Bully Hill ore yielded 76 per cent. This relation appears to carry through all of the experimental work done; a high iron content seems essential to the formation of large amounts of zinc sulfate in spite of the fact that, during that part of the roasting period when the zinc sulfate is formed, nearly the same proportion of the remaining zinc sulfide is converted to sulfate.
Zinc sulfate decomposes when it is heated above 575° C., the rate of its decomposition increasing rapidly as the temperature is increased. This is clearly shown in Fig. 4 and agrees with the known chemistry of zinc sulfate. Woehler and Plueddemann studied the decomposition pressures of zinc sulfate heated to various temperatures in closed vessels and Mostowitsch studied the decomposition of zinc sulfate heated to various temperatures in dry air currents. Mostowitsch showed that zinc sulfate heated in the presence of iron oxide decomposed more rapidly than zinc sulfate heated alone, which indicates a definitely exothermic reaction between zinc oxide (or sulfate) and iron oxide to form ferrites. He further showed that the presence of silica did not accelerate the decomposition rate of zinc sulfate until the temperature exceeded 1000° C., indicating that zinc silicate is not formed below that temperature. The data of Wochler and Plueddemann and of Mostowitsch have been plotted in Fig. 14.
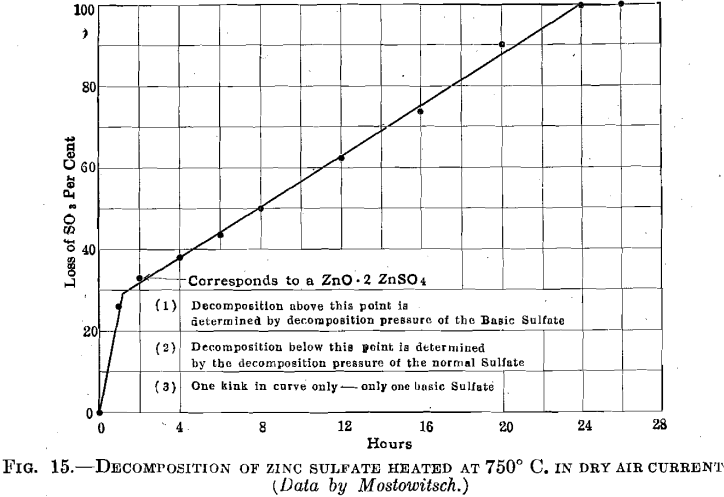
Mostowitsch further showed that the breaking up of zinc sulfate at 750° C. required 24 hr. for its completion. It is not, then, a rapid reaction at such temperatures we are concerned with. His data, plotted in Fig. 15, are consistent with the existence of one, and only one, reaction product intermediate between zinc sulfate and zinc oxide, and the
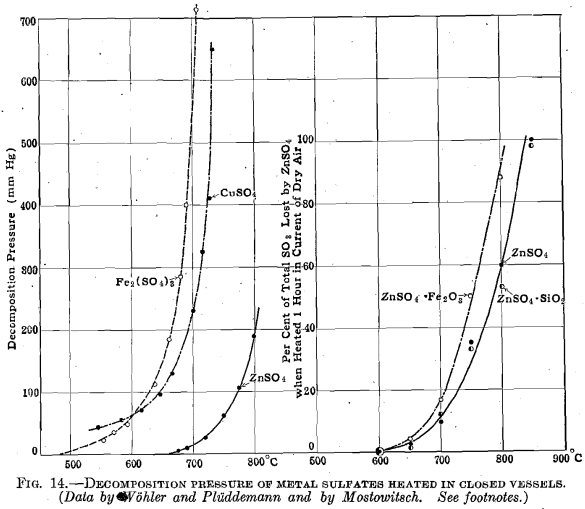
break in the curve indicates this to be a basic zinc sulfate of the composition ZnO:2ZnSO4. This same compound, in hydrated form, is found in the zinc leaching plant, where it is distinctly disadvantageous. It is formed by the direct solution of zinc oxide in neutral zinc-sulfate solution; it is formed through the precipitation of zinc hydrate when lime is added to coagulate the leaching solutions and by the action of metallic zinc upon zinc-sulfate solution with the liberation of hydrogen during the purification processes. That basic sulfate is to be found in roasted calcines is at times indicated by the presence of sulfur, which is insoluble in water but readily soluble in dilute hydrochloric acid. The highest values found for such basic sulfate sulfur were determined in Bully Hill calcines, which contained 0.74 to 0.89 per cent.
Formation of Zinc Ferrites
The writer uses the term “ferrites” from mere habit. Hamilton, Murray, and McIntosh use the more chemically correct term “ferrates” in describing work they did at Trail in the way of synthesizing ferrates by heating together intimately mixed portions of pure zinc and iron oxides. By heating molecular proportions of
zinc oxide and ferric oxide at various temperatures, they found that the zinc oxides became insoluble. The following data are interpolated from their plots:

From similar experiments, in which the proportions of zinc oxide and ferric oxide were varied, they concluded that no ferrates were formed that contain more zinc than ZnOFe2O3, but that when these oxides are heated for any length of time above 650° the iron will render one-half its equivalent of zinc insoluble. They also give the results obtained in heating thirteen various ores in a small hand-rabbled muffle furnace at 750-800° C. They found that the majority of the ores yielded calcines in which the insoluble zinc and total iron bore simple molecular ratios, but that some of the calcines, notably those from the Sullivan ores, carried much less insoluble zinc than was to be expected from their iron content. Their explanation is of decided interest.
“In some way, therefore, the combination is prevented from being as complete as one should expect from the composition of the ore. One explanation may be that at this concentration of the zinc and iron another compound may be formed than that represented by the formula ZnO-Fe2O3, but we have been unable to prove this and regard the existence of any combination except ZnOFe2O3 as unlikely. On the other hand, it may be that free crystals of pyrrhotite do not combine as readily with those crystals which are directly attached to crystals of blende, or as that iron which may be isomorphous with the zinc in the blende.”
The experimental data presented here were selected to cover the various ore types mentioned, and it seems that the experimental results leave little doubt as to the correctness of the above explanations.
The Daly Judge ores contain pure blende and pure pyrite, so coarsely crystallized that the crystals can be separated and analyzed to make sure that the zinc and iron are not isomorphous. Furthermore, the minerals are so coarsely crystallized that the major fraction of the particles of concentrate roasted are either blende or pyrite and seldom part one and part the other. The concentrates were coarse which made it necessary to roast them for long periods at temperatures well above the known formation temperature of the ferrates. In spite of this, the resulting calcines contained but from 3.2 to 14.1 per cent, of the insoluble zinc called for by the iron content and the ferrate formula.
Bully Hill ore is a massive sulfide in which the minerals are so finely divided and intermixed that it is practically impossible to identify blende or pyrite. But the ore contains sufficient sulfur to account for the iron as pyrite and sulfur is volatilized in roasting. This is presumptive evidence against isomorphous crystallization since the isomorphously crystallized iron is invariably FeS rather than FeS2. The ore, however, is so finely crystallized that the average roaster feed particle must contain both blende and pyrite attached to one another. The careful roasting of Bully Hill ore below the formation temperature of ferrates led to 93.6 per cent, zinc extractions, leaving insoluble zinc in the calcines amounting to but 15.7 per cent, of that called for by the iron content and ferrate formula. Roasting for long periods at 700° C. led to 65 per cent, extractions and to calcines containing 86 per cent, of the insoluble zinc called for by the iron and ferrate formula. It is probable that the calcines contained some sulfide sulfur, which would still further reduce the amount of insoluble zinc accounted for as ferrates in the above.
Broken Hill concentrates appear to consist of isomorphously crystallized zinc and iron sulfides. No pyrite is obtrusively visible and the concentrates do not contain sufficient sulfur to account for the iron as anything but FeS. No manner of roasting, either below or above the formation temperature of ferrate, led to extractions higher than 87-88 per cent., and the same extraction was invariably obtained so long as the sulfides were oxidized. The amount of insoluble zinc left in the calcines is slightly greater than that called for by the iron content.
The Frisco concentrates appear to be made up of isomorphously crystallized iron and zinc sulfides, and the highest extraction obtained still left in the calcines 80 per cent, of the insoluble zinc called for by the ferrate formula.
The formation of ferrates can take place in but two ways: The reaction takes place between two solid oxides or one (or both) of the oxides must be sufficiently volatile to distil into contact with the other. It seems more probable that the first is the more important and that unless the iron and zinc are isomorphously crystallized there must be mutual diffusion of one oxide into the other before the reaction can take place. Diffusion is, at best, an extremely slow process, hence ferrates are formed readily only when the zinc and iron are isomorphous. When blende and pyrite are firmly attached to one another there is, at least, a common contact plane through which diffusion can take place. Probably no ferrate is formed if the temperature is kept sufficiently low, and more ferrate is formed as the temperature is raised and the diffusion rate is increased. Ores of this type, like the Bully Hill ore, can be commercially roasted to yield far better extractions than ores containing similar amounts of zinc and iron that are isomorphous.
Finally, when the roaster feed is made up mainly of separate particles of blende and pyrite, as in the case of the Judge concentrates, and possibly in the case of the Butte Superior concentrates, there are only casual contacts between the iron and zinc particles through which diffusion can take place and the formation rate of ferrate is so low that it scarcely enters into the roasting problem at all.
Solubility of Iron in Roasted Calcines
Much dire distress was predicted in connection with the leaching of ferruginous calcines. Iron is very readily oxidized and the trouble in the leaching plant has been due to leaching too little iron rather than too much. At Bully Hill, the average leaching solution, made up to 100 gm. zinc per liter by leaching calcines with acid and water, contained 0.020 gm. of iron per liter. Later on, when it was found that dissolved iron was essential to the complete removal of arsenic and antimony, we tried to so roast the ore that more iron would dissolve; to use such acid concentrations as would lead to dissolving more iron; and finally purchased iron and iron salts as being the cheaper alternative. The solubility of iron at various stages of the roasting process is indicated by the following data taken from the Wedge furnace at the Judge plant (experimental series 1, Table 2):

Behavior of Manganese
Practically all the manganese dissolved in the leaching plant is present in the calcines as manganese sulfate, which is stable at higher temperatures than zinc sulfate. Ordinarily, about 10 per cent, of the manganese present in the calcines will be dissolved in the leaching plant.
Behavior of Silica
A bugaboo in the shape of “colloidal silica” has traveled about the various electrolytic-zinc plants and great importance has been attached to tracing it to its source. The natural oxidized zinc ores (oxide, carbonate, and hydrated silicate) frequently do give trouble in the leaching plant since considerable amounts of silica can be dissolved from them and it requires but 2 or 3 gm. of silica to gel a liter of solution. All siliceous calcines worked with have yielded very small amounts of silica to the solution and it does not appear that the amount of silica dissolved bears any relation to the source of the roaster feed, nor to its total silica content. Trouble from dissolved silica has frequently been traced directly to the lime or limerock used for neutralizing and coagulating the leach solution; probably this is the more frequent source of trouble.
Behavior of Bases
The acid consumption in leaching various calcines makes it appear that both lime and magnesia are converted to sulfate in the roaster. In no case has it appeared that these bases consumed acid in the leaching plant. Magnesium sulfate is a cumulative impurity in the zinc-plant solutions but it has not been of any importance in. any instance as yet on record.
Behavior of Lead
Lead sulfide is converted to lead sulfate during the early stages of the roast and is left in the calcines as sulfate, since the lead can be almost completely extracted from either the calcines or from the leach-plant residues with strong sodium-chloride solution. It does not appear that any insoluble lead-zinc compounds are formed but there is a very marked tendency for high lead concentrates to sinter in the early stages of the roasting process.
Behavior of Arsenic and Antimony
Both arsenic and antimony are volatilized in roasting, but not so completely as to render them unimportant in the calcines. In roasting ores from the North Star mine (Hailey, Ida.) in the small electric roaster, the outlet air tube became choked with arsenic trioxide shortly after the roasting had started, and further evolution of arsenic soon ceased.

Antimony appears to be less completely volatilized than arsenic. From the operating standpoint, it matters little whether the roaster feed carries a fair fraction of arsenic and antimony, or whether it carries a barely detectable trace of them. In either case enough arsenic and antimony will be dissolved to cause need for their removal from the leaching solution.
Behavior of Copper
Ordinarily the roasting temperature used in roasting for zinc extraction is too high to yield a high copper extraction. As much as 50 per cent, of the total copper has been dissolved in regular practice, but the average is probably nearer 20 per cent.
Thanks are due Mr. G. W. Lambourne and Mr. Oscar Friendly of the Judge Mining & Smelting Co. for permission to use the data collected at Park City, and to Mr. E. H. Hamilton for his suggestions and editing of this manuscript.
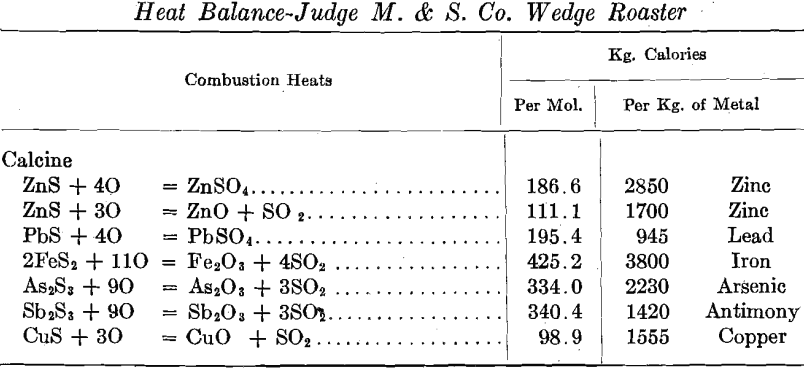
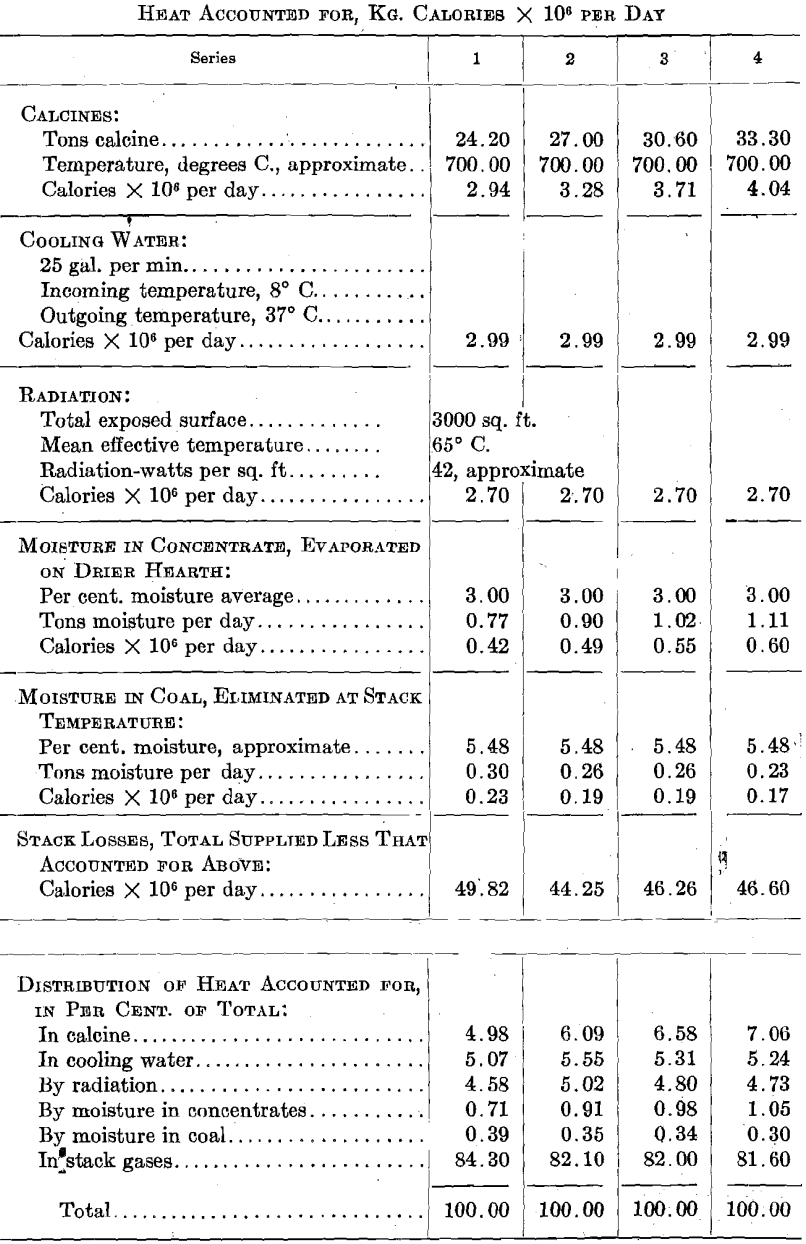
Gas Volumes, Gas Temperatures, Stack Heat Losses
As previously stated, the pyrometer couples in this roaster were installed in grooves cut into the upper surface of each hearth. The temperatures reported throughout are calcine temperatures rather than gas temperatures. The following comparison of gas and calcine temperatures covers one day’s operation under uniform conditions: the usual pyrometer installation is made with the thermocouples so arranged as to measure gas temperatures rather than calcine temperatures. It is the calcine temperature which determines ferrite formation, and not gas temperature directly. We had trouble with the couples placed in the hearth in that as the hearth raised the couples were pried out and caught by the rabble blades.
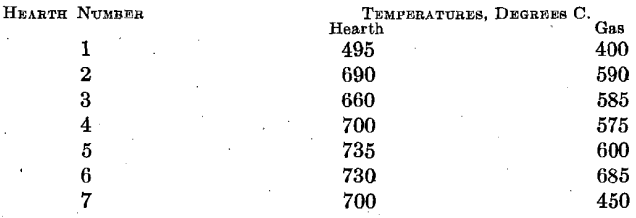
This is the only direct comparison at present available and in that which follows it is assumed that the difference between calcine temperature and gas temperature remains as above.
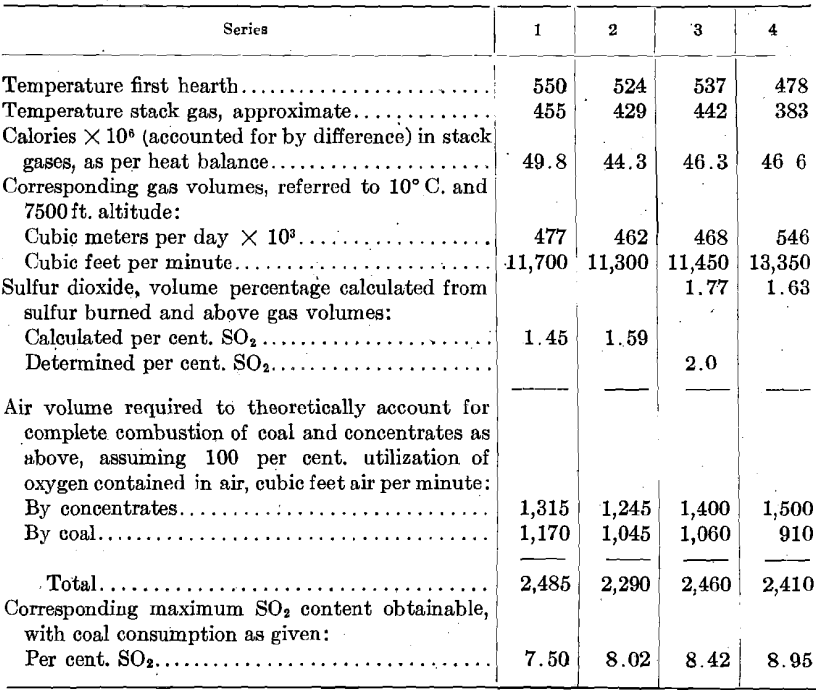
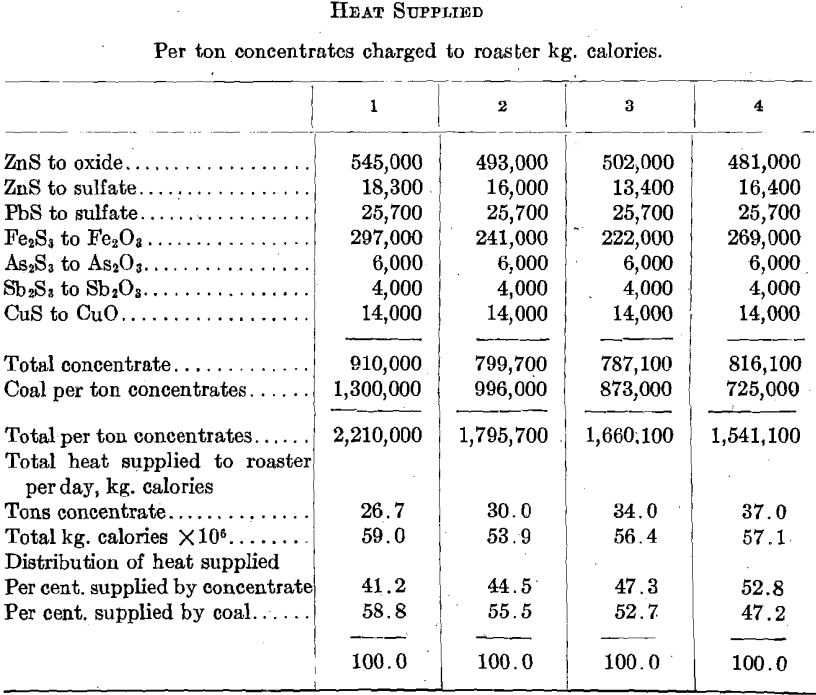
The agreement between the calculated sulfur-dioxide concentrations in the roaster gas and the determined concentrations is sufficiently close to fix the air-feed volume between 10,200 and 11,450 cu. ft. (285 and 320 cu. m.) per min. for series 3. It is to be noted that the mean of these volumes [10,825 cu. ft. (303 cu. m.) per min.] is something like 7.8 times the air volume theoretically required for the oxidation of the concentrates, and something like 4.4 times as great as the air volume theoretically required for the concentrates and for the coal actually consumed.
More than 80 per cent, of the heat supplied to the roaster is accounted for in the stack gases, and it would appear that a very considerable reduction in coal consumption could be brought about by reducing the amount of air fed to the roaster without materially affecting the operation of the furnace. With the furnace arranged as at present any additional air fed to the furnace must be heated to the stack temperature (at least) through combustion of coal and at the expense of oxygen contained in that air. It does not appear at all impossible that the use of an excessive volume of air might actually lead to lower oxygen concentrations . in the roaster gas, and thus to lower oxidation rates and to lower roaster capacities.
The following calculations, based on the roasting of 30 tons of concentrates per day, as in series 2, are at least interesting in that they indicate some possibilities of air volume control.
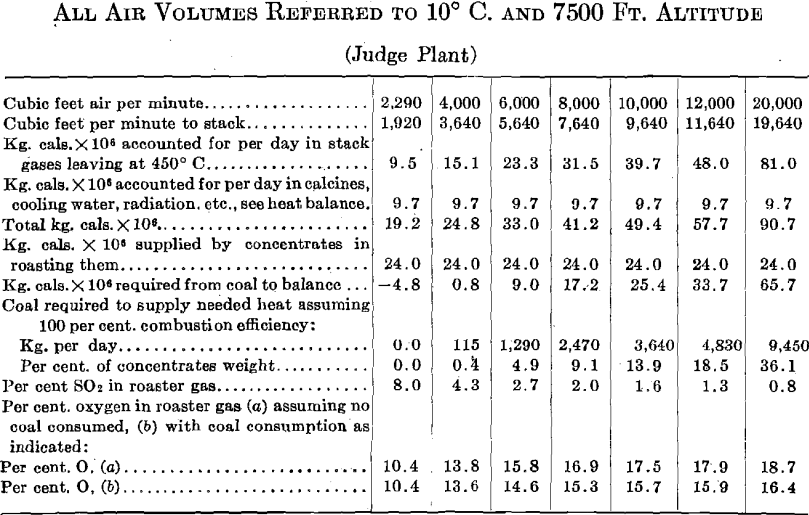
If we assume that the oxidation rate, and therefore the roaster capacity, is proportional to the oxygen concentration, and this is an ultra-conservative assumption in this case, the relation between air volume, roaster capacity, and fuel consumption must be something like the following:
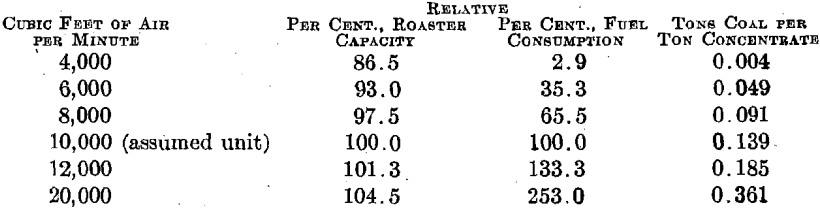
It appears quite certain that there is more to be gained in fuel economy through careful study of air volumes than there is to be gained in greater roaster capacity through the use of excessive air volumes.
The Wedge type of furnace was probably developed to serve the particular purpose for which it is mainly used, that is for the incomplete combustion of sulfides without need for outside fuel, rather than for such complete combustion as is necessary in roasting zinc concentrates. There is little or no indication that this type of metal melting furnace cannot be well adapted for the roasting of zinc sulfides, as well adapted perhaps as any other type of furnace, but it is possible that it will require considerable study and, possibly, some special development of the furnace and practice, before completely satisfactory and most economical operating conditions are both realized.
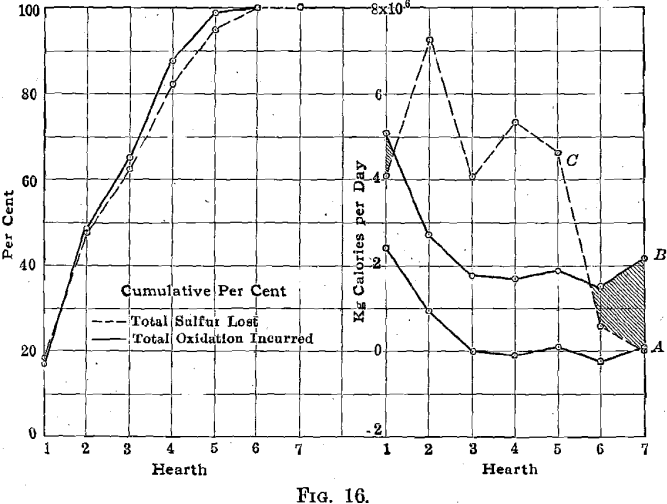
The furnace is at present updrafted, and the calcines pass downward through the furnace. The major fraction of the heat evolved by the burning sulfide is liberated in the upper parts of the furnace, is imparted to the roaster gas, and is carried on upward through the furnace. In the lower parts of the furnace, insufficient heat is evolved to maintain the temperature required for complete combustion in reasonable time. This is brought out in Fig. 16, where curve A indicates the amount of heat absorbed by the calcines (weight, specific heat, and temperature of the calcines); curve B indicates the total amount of heat accounted for on each hearth in cooling water, by radiation, and absorbed by the calcines; curve C indicates the amount of heat evolved on each hearth by the combustion of the sulfides. The shaded area between curves B and C represents an amount of heat that must be supplied merely to maintain the roaster temperature, and the unshaded area between the same two curves represents heat that should be available for supplying the deficiency on the lower hearths and for heating the roaster gases, without resort to the use of coal or other fuel.
It is clear that so long as the furnace is updrafted there is no possibility of utilizing the excess heat in the upper parts of the furnace to make up the deficiencies in the lower parts of the furnace. That deficiency, which must be made up through the use of other fuel, amounts to 3,000,000 kg.-cal. per furnace per day and is made up almost wholly of cooling water losses and radiation, both of which can be reduced somewhat. In addition (at the Judge plant, and it is the usual arrangement), some 5000 cu. ft. per min. of air, used in cooling the upper rabble arms and thus heated to 165° C., is discharged into the fifth hearth chamber and must be heated to about 600° C. if it is not to cool that hearth materially. The heat required to heat this air is supplied by the combustion gases from the coal-fired grates; and in order to effect the 600° C. average temperature in the hearth chamber these combustion gases are actually maintained at nearly 1400-1500° C. (as measured in the flues that connect the coal-fired combustion chambers with the roaster). Less than 5 per cent, of the oxygen required for the combustion of the sulfides is consumed on the lowest three hearths, and evidently only a very few hundred cubic feet of air per minute would be sufficient for this purpose. The excessive amount of air introduced into the fifth hearth chamber, however, makes it necessary either to add very much larger quantities of hot combustion gases in order to heat this air, or else to add smaller quantities of combustion gas heated to excessive temperatures. Under the conditions maintained while the experiments were in progress, the temperatures in the combustion gas flues were such that only silica brick would stand up, and it is evident that one cannot get much further on by heating still smaller combustion gas quantities to still higher temperatures. It would seem that better results could possibly be obtained by either discharging a fair part of the air used for cooling the rabble arms outside the furnace or, at least, higher up in the furnace.
Again, the major item of upkeep expense of the roaster has certainly been occasioned by the extremely hot gases entering the roaster from the firebox flues. It would not appear at all impossible that much of this trouble could be done away with, and that the roaster could be made virtually independent of outside fuel, by downdrafting all the furnace, or at least the lower five hearths. To do this would probably require exhaust fans, since otherwise noxious gases would probably fill the roaster building whenever a roaster door was opened; but this does not seem to be a very decided disadvantage. Neither does it appear impossible to substitute electrical energy for fuel firing, with the furnace otherwise unaltered as regards the draft arrangement. At most, it would require the supply of some 3,000,000 kg.-cal. to the roaster per day at a temperature potential well within the reach of metal heaters. This amounts to a continuous load of some 145 kw. per furnace, and this could certainly be reduced if a little attention were paid to the rabble arms and furnace walls of the lowest hearth chamber. An electrolytic-zinc plant must in any case have cheap power, say 0.4 to 0.5 c. per kw.-hr. Good coal scarcely exists in the districts where electrolytic-zinc plants appear to have their most promising future, and even poor coal in some of these districts is very expensive. The writer has in mind one prospective plant for making 150 tons of spelter per day where coal yielding 8000 B.t.u. per lb. will cost $12 to $15 per ton, and where power will not exceed $15 per hp.-yr. The use of power, say at the rate of 100 to 200 kw. per furnace, with a furnace capacity of 30 to 40 tons per day is by no means out of question as regards the relative costs of power and coal, and it would appear possible that the use of power would lead to a more uniform roaster practice.
Again, the writer has in mind several interruptions of service at the Judge plant during recent winters through failure of the coal supply and through failure of the transport service between the coal mine and the zinc plant coal bunkers. The question of fuel economy and of the possible substitution of power for fuel is not, then, so far fetched as it might appear, and it has led to the building of a small electrically heated roaster with which it is hoped that some of these possibilities may be explored.
