The difficulty of roasting arsenical and antimonial ores may be avoided by taking ore, 1 A.T. ; red lead, 1,000 grains; sodium carbonate, 500 grains; potassium ferrocyanide, 550 grains, with a cover of salt or borax. The button is scorified, together with the matte formed, before cupellation. E. A. Smith found this method unsatisfactory and recommends the oxidation of sulphide of antimony by nitre, the charge being made up as follows in the case of an ore containing 75 per cent, of stibnite:

With cover of powdered salt. The fusion is effected in large crucibles at a low temperature. Carbonate of soda forms a fluid slag with oxides of antimony. It is perhaps better to scorify these ores as well as those containing much copper if they are rich enough.
Metallurgists recommend that ores consisting chiefly of sulphide of antimony should be mixed with twice their weight of silica, and roasted at a very low temperature. The sand prevents the charge from “balling.’’ Sulman prefers to mix the charge with carbon instead of sand. In roasting this mixture in a reducing atmosphere, some 96 or 97 per cent, of the antimony is volatilised as sulphide at a fair red heat without loss of gold.
If they are poor, ores containing copper may be treated in three ways,; so that each method may serve as a check on the others:
- (a) Fusion with much PbO : the lead button becomes cupriferous, and should be scorified together with the matte;
- (b) roasting, followed by fusion and scorification;
- (c) treatment with nitric acid, by which all the sulphur and copper are removed.
The silver dissolved in the liquid is then precipitated by a solution of common salt, of which a large excess should be avoided. The insoluble residue is dried, and can now be readily fused and cupelled. By treatment (c) the lead button is kept free from copper, the presence of which in the lead obtained by methods (a) and (b) renders cupellation difficult and unsatisfactory.
Zinc ores may be roasted, and fused with a somewhat larger quantity of borax and sodium carbonate than usual with pyritic ores. Instead of roasting, it is easier to desulphurise blende with metallic iron in the fusion, the zinc being volatilised. Lead ores should not be roasted, but fused with plenty of iron. E. A. Smith recommends the direct fusion of bismuth ores with comparatively large quantities of sodium carbonate and borax, using a low temperature.
Telluride ores are not roasted but fused with excess of litharge in a moderately hot fire. Charges for two siliceous American ores containing tellurides are given as follows:
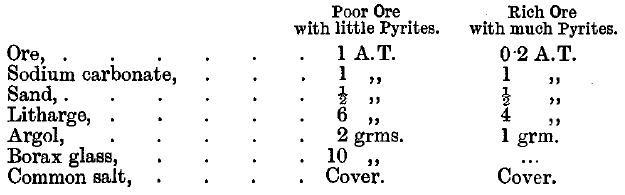
The slag is remelted with 1 A.T. of litharge and 2 grammes of argol. The lead button is cupelled direct. Tindall points out that a most important point in the assay of telluride ores is to crush the ore finely. Cripple Creek ores are crushed through 120-mesh sieves if they are poor. Richer ores are crushed through 150-mesh sieves, and very rich ores through 200-mesh sieves. Lodge states that in Cripple Creek a flux is used made up as follows:
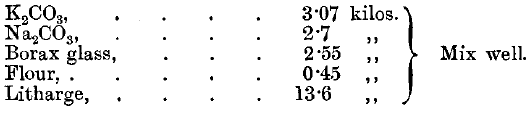
Take about 65 or 70 grammes—that is, 2 1/3 A.T.—of this mixture to ½ A.T. of ore, and fuse in a muffle furnace. The slag will be glassy and brittle.
Hillebrand and Allen found that the crucible assay is perfectly satisfactory for telluride ores from Cripple Creek. The charge giving the best results on ores containing from 15 to 20 ozs. of gold per ton was as follows:

The losses of gold in the slag were generally small. The losses by cupel absorption were much more serious. On the other hand, E. C. Woodward found that the cupel losses were small, never rising above 1 per cent, of the gold. J. C. Bailar records cupel losses ranging up to 100 per cent. Further investigation is evidently required. For assay of tellurides by scorification.
Roasting before Fusion & Assay
Ores containing large quantities of sulphur, arsenic, or antimony may sometimes be roasted with advantage as a preliminary to fusion. Roasting is effected in shallow circular clay dishes, in a muffle, or in the crucibles in which the fusion is afterwards performed. The temperature must be kept low at first and the ore frequently stirred with an iron wire or spatula, to prevent fritting, and to expose fresh surfaces to the air. The roasting takes place in two stages : at first, sulphur dioxide, arsenious oxide (As2O3), and antimonious oxide (Sb2O3) are formed and volatilised, the sulphur burning with a blue flame. The formation of lumps is most to be feared during the first few minutes of the operation, and can scarcely be prevented if much sulphide of antimony is present; in this case an equal weight or more of pure silver sand is mixed with the crushed ore before charging it into the muffle.
After a time the blue flame disappears, the odour becomes less strong, and sulphates, arseniates, and antimoniates form. By raising the temperature sulphates are decomposed, but arseniates and antimoniates are stable at high temperatures and cause loss of silver in the fusion. To prevent their formation the ore should be roasted in a coke furnace, starting to heat it very gradually and admitting a limited supply of air. In all cases the roasting is nearly complete when the glow caused by stirring is shown only by a few specks of ore ; the temperature may then be raised to a strong red heat without danger of fusion. The operation is complete when the ore remains of a uniform colour on stirring. Arseniates and antimoniates may be in part removed by re-roasting with powdered charcoal in a covered dish. The fusion of roasted ores requires more charcoal powder than raw ores, the amount needed being sometimes as much as 3 grammes per A.T. of ore. In general, roasting is to be deprecated, owing to the unavoidable loss by dusting. The results are usually lower than those obtained by other methods, and the operation requires the expenditure of much time and attention. It is useful as a second method in cases of special importance.
