Selenium (Se) is encountered in surface and groundwaters in the United States at concentrations that rarely exceed the drinking water standards (0.01 mg/L). However, in the western United States, leachates from heap-leaching operations at gold mines may contain selenium concentrations as high as 2 mg/L. In the gold heap leaching process, a solution containing sodium cyanide is sprinkled on the heaped ore to extract gold and silver. Other metals, including selenium, are leached in the process. At closure, the mines are required to detoxify the spent ore piles. This is normally accomplished by rinsing the spent ore with fresh water. The leachate generated from this rinsing must be treated prior to its final disposal. Regulatory agencies typically require that selenium be removed from the leachate to 0.1 mg/L or 10 times the drinking water standards.
In aqueous systems selenium exists in two predominant oxidation states: selenium IV (selenite) and selenium VI (selenate). Coagulation and softening have been used to remove selenium from drinking water supplies but the removals generally have not been satisfactory (Sorg, 1978). Clifford (1989) cites studies of ion exchange resins used to remove selenium in which Se (VI) was better adsorbed by ion-exchange than was Se (IV). Yuan (1984) demonstrated that both Se(IV) and Se(VI) can be removed effectively by activated alumina and that the adsorption process is highly dependent on pH. The adsorption of selenium (VI) continuously decreased with an increase in pH and almost 100% of Se (IV) was adsorbed at pH < 8.0 (Yuan, 1984). To the best of our knowledge, published studies on selenium adsorption by activated alumina have been performed using only relatively “clean” drinking water supplies or synthetic selenium solutions. The results of these studies cannot be directly applied to the treatment of complex wastewaters, such as mine leachates, in which other ions compete for the alumina adsorption sites. Consequently, a study of the adsorption of selenium from a complex mine leachate was conducted to develop design parameters for full scale activated alumina adsorption processes.
The following conclusions were drawn from this study:
- The investigation of the selenium speciation of the mine leachate revealed that the leachate typically contained 0.95 ppm selenite, minor amounts of selenate, and 0.56 ppm of an unidentified selenium compound. The study also revealed that some ions such as sodium (Na+) and sulfate (SO4-²) were present in the leachate at very high concentrations.
- It was possible to remove approximately 68 % of the selenium contained in the mine leachate by using activated alumina as the adsorption medium. The remaining 32 % consisted of an unidentified selenium species which could not be adsorbed.
- Batch tests indicated that there was no significant difference in selenium adsorption when using Discovery DD6 alumina, Alcoa F1 alumina, and La Roche A-1 alumina.
- Not only selenium, but also the silica (24 mg/L as Si) contained in the leachate was adsorbed by activated alumina. This silica competed for adsorption sites and reduced the selenium adsorption capacity of the aluminas.
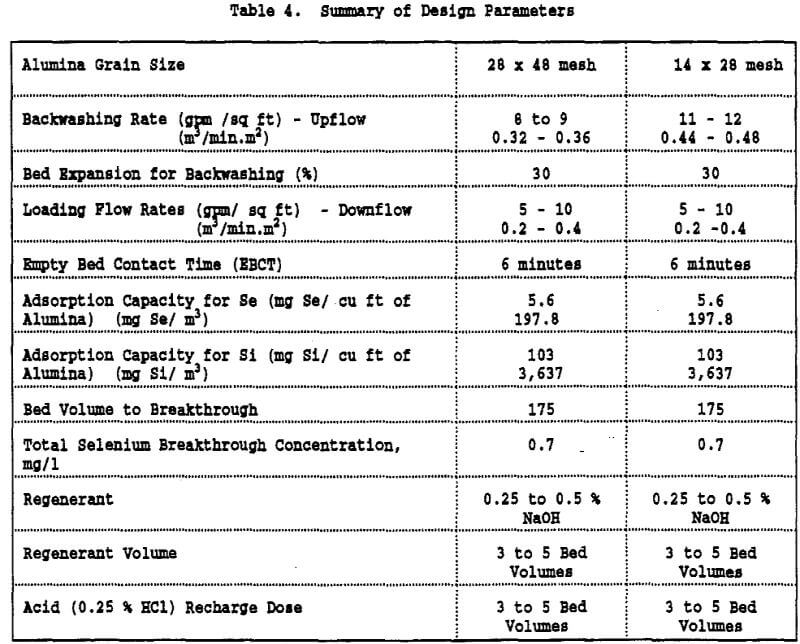
- The selenium adsorption capacity for pH values ranging from 6 to 9 was 5.6 g of Se/ cu ft of alumina while the capacity for silica was 103 g Si/cu ft of alumina. For pH < 4.0, silica was poorly adsorbed and the adsorption of selenium was somewhat improved. However, the improvement in selenium adsorption may not justify the costs associated with working at very low pH values. In addition there is a safety issue associated with decreasing the pH of cyanide-containing solutions.
- Selenium and silica could be desorbed from the activated alumina by using 0.25 % to 0.5 % NaOH solution. The volume of NaOH required to regenerate the spent alumina was estimated as 3 to 5 BV.
- Critical parameters were determined for designing a full scale alumina adsorption plant for removing selenium from leachate. These parameters are compatible with similar designs presently in full scale operation, except for the alumina adsorption capacity (5.6 g Se/ cu ft alumina) which was much less than the capacity obtained for synthetic selenium solutions (24.4 g Se/ cu ft of alumina).
- The design of a full-scale alumina adsorption plant to treat mine leachate must take into consideration the method and cost for storing, processing, and treating the clean and spent regeneration solutions.