Table of Contents
The gold ores that are currently being processed contain mercury which is not only hazardous to the environment, but also causes serious occupational health and economic problems during gold processing. Some of the mercury present in the ore is dissolved during cyanidation of gold. As the separation of mercury cyanide from gold cyanide is difficult, it accompanies gold till the end of the processing stream where mercury is finally recovered by distillation. Since volatilization of mercury occurs at each stage of processing such as carbon stripping, carbon regeneration and electrowinning, and mercury vapor is extremely toxic, it poses serious worker health concern due to vapor exposure. In addition, the economics of the gold production process is also affected due to additional reagent consumption as the leached mercury competes with gold during adsorption on to activated carbon, reduced efficiency of Merril-Crowe process and extra capital and processing costs for mercury control and removal.
Even the unreacted mercury left behind in the abandoned tailing impoundment may create serious environmental problem. Although considered stable, the volatilization of Hg° from cinnabar by T. ferro-oxidants is possible. Also, methylization of mercury by sulfide reducing bacteria produces a more toxic and biotically available form of mercury.
In view of the above mentioned problems and the stringent environmental regulation on heavy metals which has set the current mercury discharge limit to 10 ppb, a suitable process for removal of mercury from gold processing stream is of utmost importance. Although several methods for removal of mercury have been investigated, no satisfactory method is yet to evolve. Most notable methods among those studied are; cementation of mercury from leach solutions by iron, zinc or aluminum addition, use of crumb rubber, selective solvent extraction of gold, and precipitation of mercury by inorganic sulfide addition. Among the above mentioned methods, mercury removal by crumb rubber appears to be a very promising one as it involves utilization of discarded automobile tires. But the technology is still in its infancy and several other aspects such as the disposal of mercury laden rubber have to be addressed before considering commercial exploitation. Other mercury removal processes suffer from drawbacks namely, formation of toxic ferrocyanide from iron cementation process, redissolution of mercury sulfide etc.
The present study was undertaken with the objective of finding a suitable reagent for the precipitation and subsequent removal of mercury from gold cyanide leach solution. Three sulfur bearing reagents, sodium sulfide, sodium and potassium dimethyl dithiocarbamates were investigated for their mercury removal efficiencies. These reagents are known for their effectiveness in removing metal ions as Na2S was found to be more suitable than lime in precipitating heavy metal ions from leach solutions and dithiocarbamates have been used for precipitation of copper and nickel from electroless effluents, preconcentration of organic mercury species from humic-rich natural waters. The dithiocarbamates also have been used for the flotation of sulfide minerals. If potassium dimethyldithiocarbamate is sprayed along with cyanide solution, the mercury can be stabilized in the heap with very minor amounts reporting in the pregnant solution.
Experiment
Two sets of tests were carried out. In the first set of tests the mercury removal efficiencies of the reagents, inorganic sodium sulfide and organic sodium and potassium dimethyl dithiocarbamates (DTCs) were compared. The second set involved the study of mercury removal from a cyanidation process water by the most suitable reagent.
Dilute stock solutions of sulfur bearing organic reagents were prepared from sodium and potassium dimethyl dithiocarbamates. Inorganic reagent was prepared from reagent grade sodium sulfide from Fisher Scientific. Reagent grade mercury nitrate also from Fisher Scientific was used for preparing inorganic synthetic solution. For preparation of synthetic solutions containing cyano-mercury complexes, reagent grade mercury cyanide from Alfa Aesir was employed. De-ionized water was utilized for the preparation of solutions.
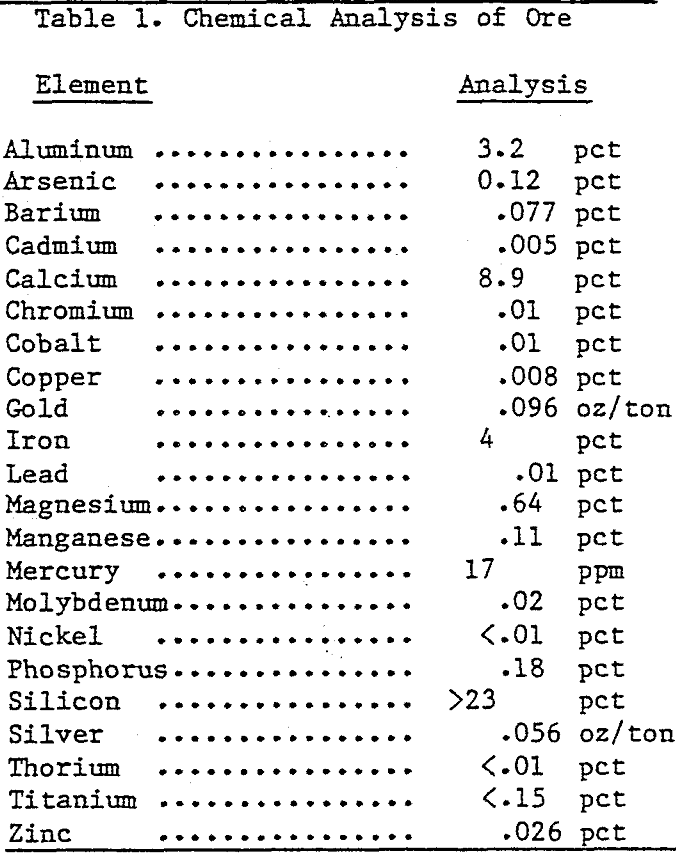
Process water containing dissolved mercury cyanide complexes was obtained from the Hollister Mines of Newmont Explorations Limited. The chemical composition of Hollister process water is given in the Table 1.
Method
About 500 ml of synthetic solution or process water was placed in a 1 L beaker and required amounts of precipitating reagents were added. The solution was then agitated with a magnetic stirrer after making necessary pH adjustments. After five minutes the stirring was discontinued. The slurry containing precipitates was filtered and the filtrate was taken for analysis.
The removal efficiencies of the reagents were determined by analyzing the initial and final mercury concentrations in the solution. Cold vapor atomic absorption technique was applied to analyze mercury in a Varian spectr AA atomic absorption spectro photometer.
The mercury removal efficiencies of Na2S and alkyl dimethyl dithiocarbamates from solutions containing mercury nitrate and mercury cyanide complexes were investigated. The results show that while Na2S can efficiently remove mercury from inorganic mercury nitrate solution, it is not as efficient in removing cyano-mercury complexes compared to alkyl dimethyl dithiocarbamates Potassium dimethyl dithiocarbamate (KDTC) was found to be very efficient in removing mercury from mercury cyanide solutions by formation of mercury dithiocarbamate complexes. About 98% of mercury from a gold mine process water containing 6.8 ppm could be removed by addition of potassium dimethyl dithiocarbamate with a reagent mercury molar ratio of 2:1. The resultant mercury dithiocarbamate complexes are stable in water and do not have any adverse influence on the gold leaching circuit either during leaching or activated carbon adsorption. Mercury can also be stabilized in the heap by the addition of KDTC during cyanidation.


 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
How to Remove Mercury from Gold Cyanide Leach Solution
During the past few years, several low-grade Au ore deposits containing Hg have been developed and are being processed by cyanidation to recover precious metals. These ores usually have less than 15 ppm Hg content. During cyanidation, 10 to 30 pct of the Hg is extracted along with Au and Ag. Gold, silver, and mercury are adsorbed from the leach solution by activated carbon in a carbon-in-pulp (CIP) circuit. The metals are stripped from carbon with hot solutions of sodium hydroxide-sodium cyanide or alcohol-sodium hydroxide-sodium cyanide.
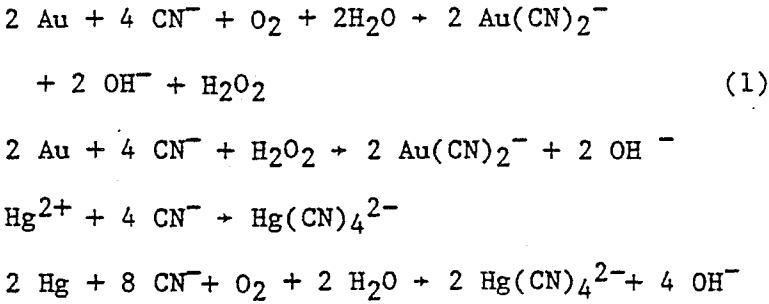
Materials and Equipment
Ore used in this investigation was obtained from an Au mining operation in northern Nevada. The ore body is described as an oxidized hydrothermal deposit with Au finely disseminated as micrometer-sized particles. Host rock of limestone-siltstone has silicified to jasperoid and partly weathered to clay. The ore was crushed and wet ball milled to give 90 pct passing through a 200-mesh screen.
Experimental Procedure Results and Discussion
A standard method of performing leach tests was developed for determining the effectiveness of Au-Hg separations in cyanide solutions using sulfides to suppress Hg solubility. Ground ore, sodium cyanide, and calcium oxide were added to distilled water in open beakers. The solution was mechanically stirred, and air was bubbled through the slurry.
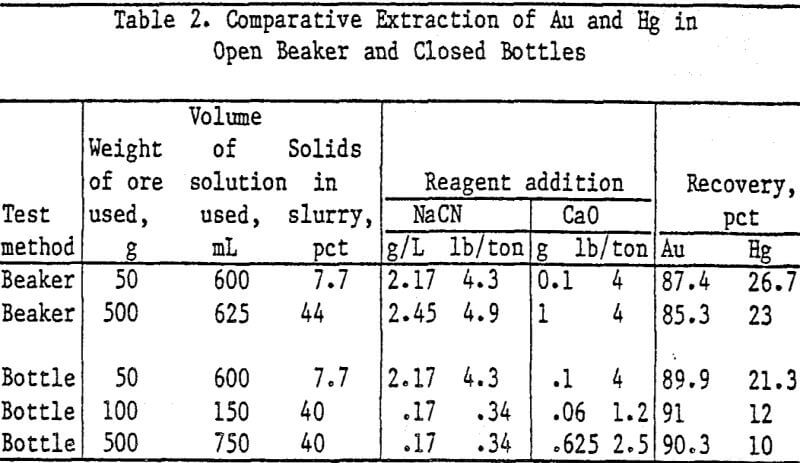
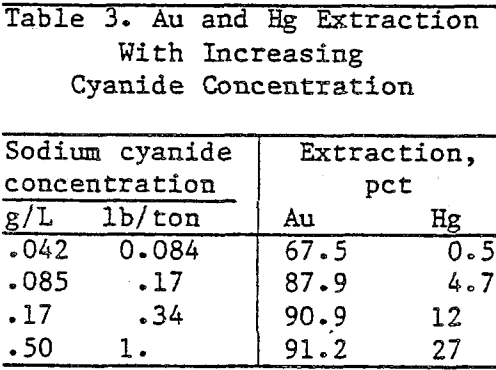
Standard bottle tests were run to determine Au and Hg extraction with increasing cyanide concentration in the leach solution. One-hundred grams of test ore were leached in 150 ml of solution containing from 0.042 g NaCN/L (0.084 lb/ton) to 0.5 g NaCN/L (1.0 lb/ton) and 0.06 g CaO (1.2 lb/ton).
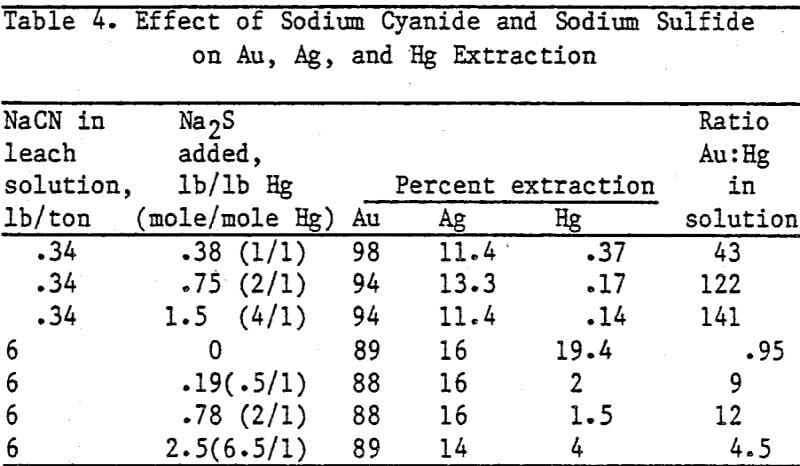
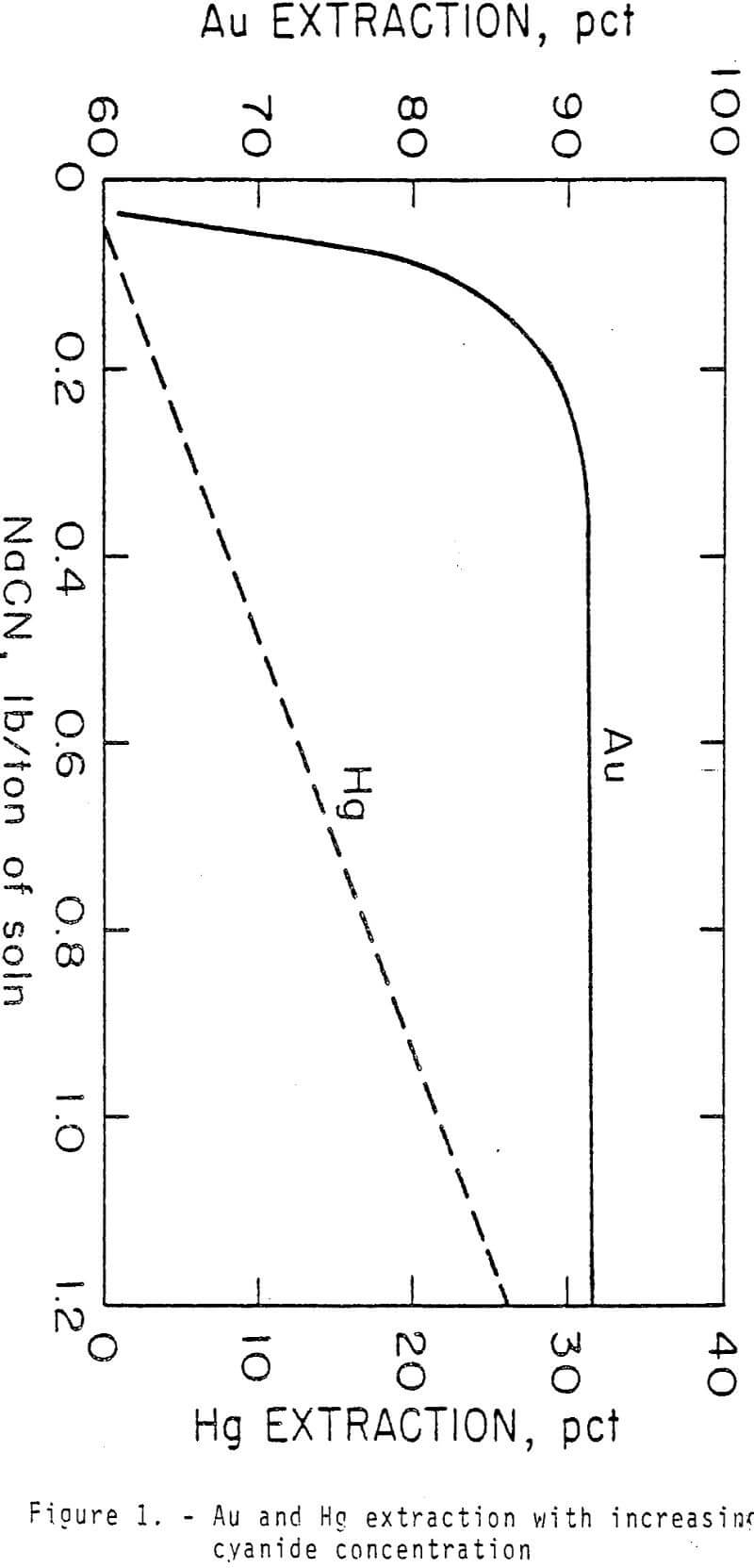
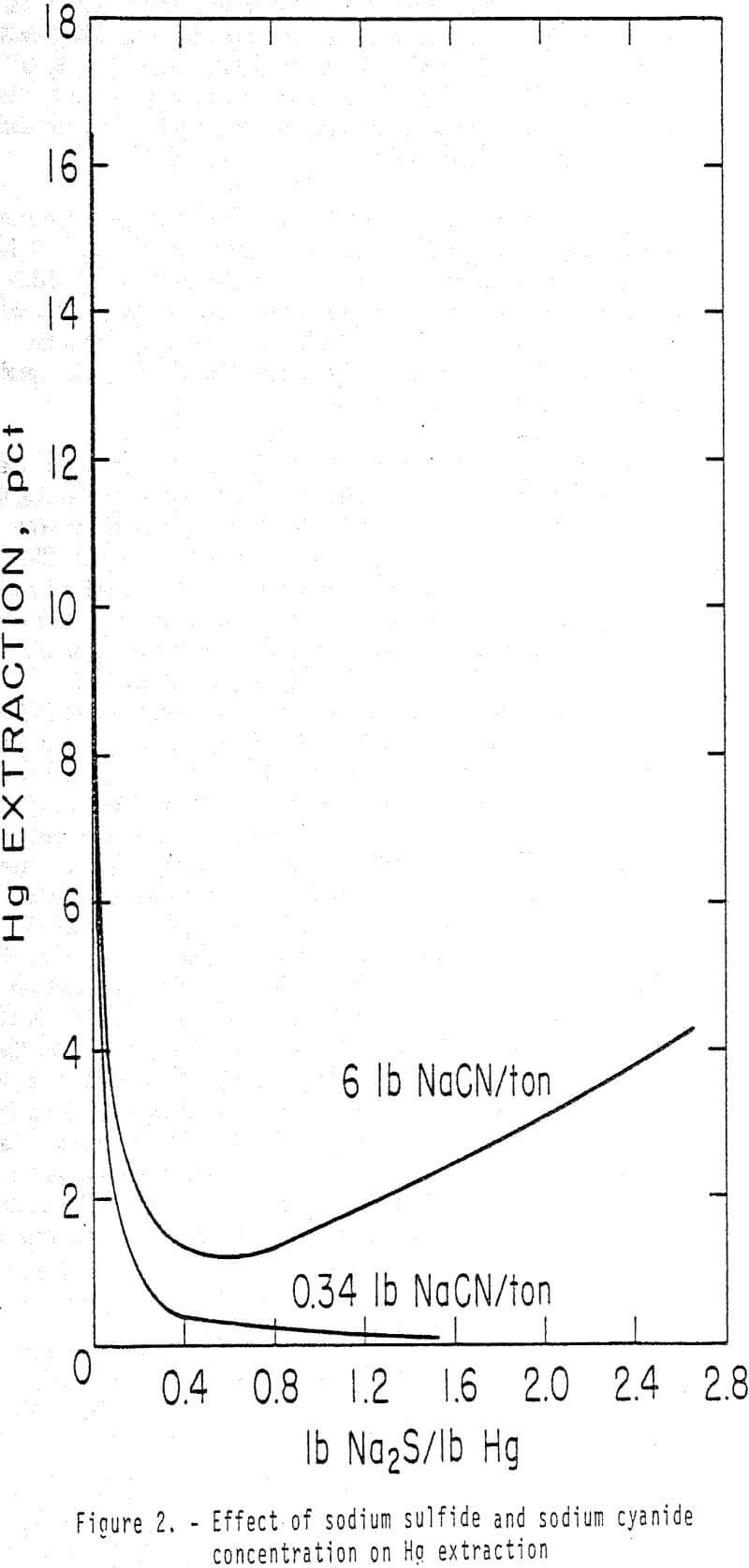
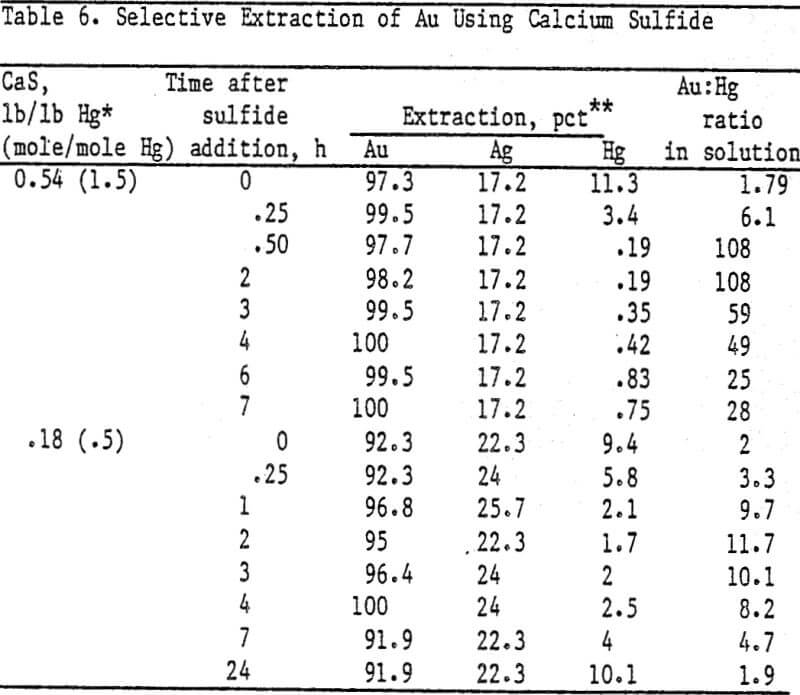
Standard bottle leach tests were conducted to determine the effect of time after adding CaS on selective Au and Ag separation from Hg. Five-hundred grams of ore were leached in 750 ml of solution containing 0.34 lb NaCN/ton solution (0.17 g NaCN/L) and 2.5 lb CaO/ton ore.
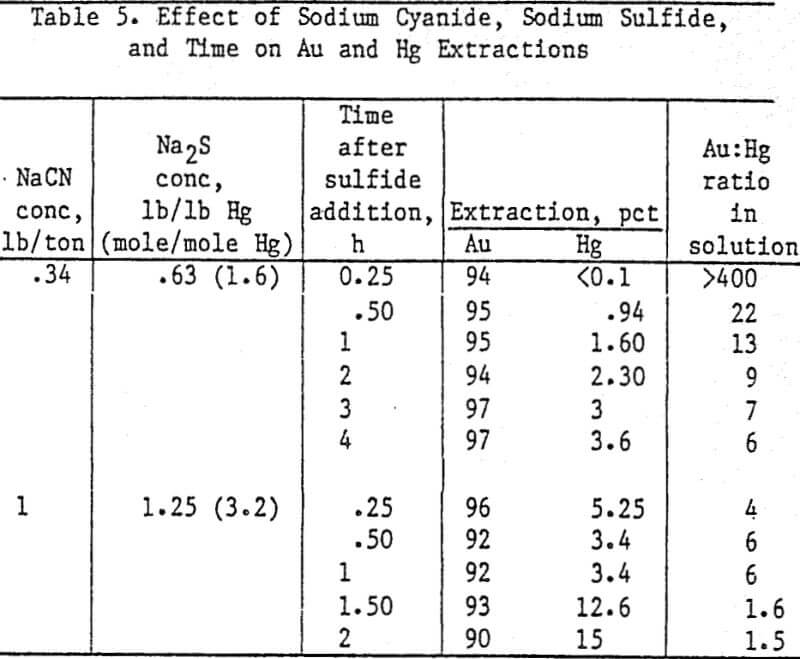
Analyses and distribution of precious metals were accomplished with radioisotopes of Au, Ag, and Hg. Radioisotopes were added to the slurry following the 24-h leach prior to addition of sulfide. Slurry samples were counted on the Gamma counter before and after adding carbon. Carbon from each stage was washed free of slurry and counted. Radioisotope counts on the head and tail slurries showed nearly 100 pct Au adsorption on the carbon; therefore Au adsorption was used as a standard for determining the percent adsorption of Hg and Ag.
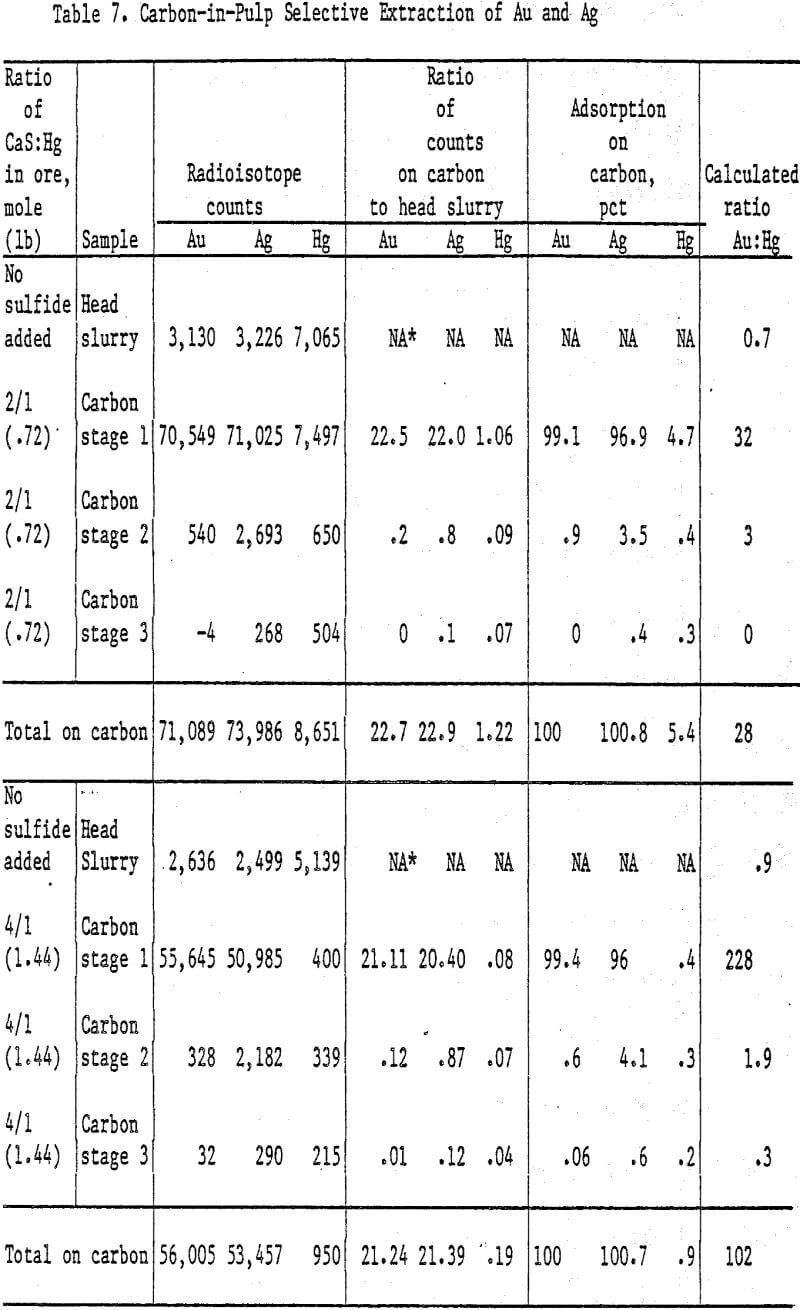
Hg adsorption on the carbon was 0.2 pct in 24 h using 2.88 lb CaS/lb Hg. More than 0.2 pet in a 6 h adsorption time, using 20 g carbon/L of slurry, would not be expected because this is the total amount dissolved in 6 h without carbon.
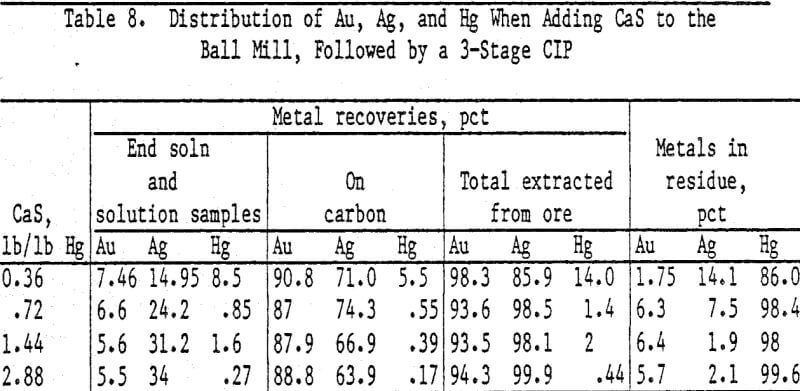
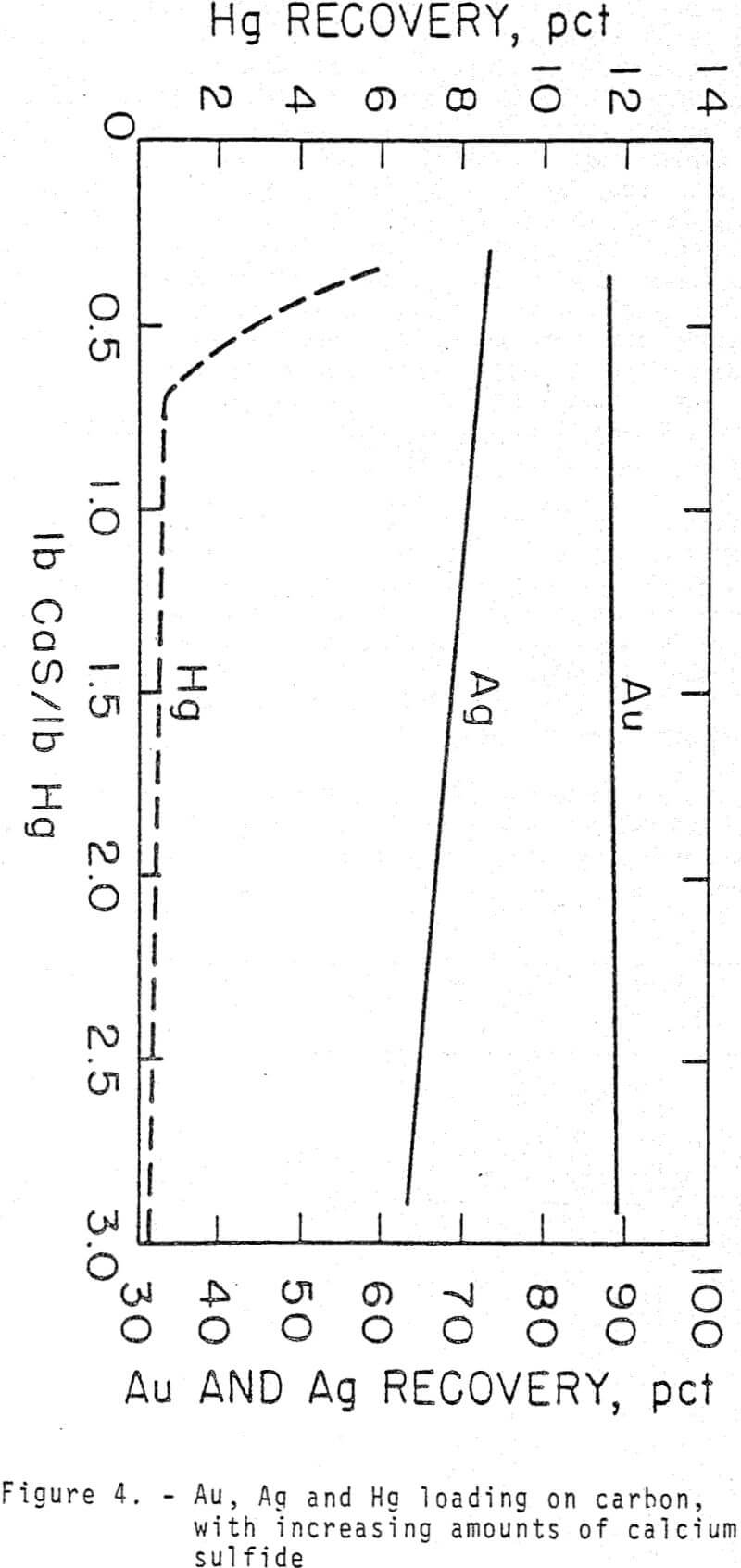
Calcium or sodium sulfides are effective in suppressing extraction of Hg without affecting An or Ag extraction in cyanide solutions. Nearly 100 pct of the Hg is rejected in the tailings with these sulfides. Calcium sulfide is preferred because less than 1 pct of the Hg was extracted from an ore in 7 h using 0.54 lb CaS/lb Hg in the ore while 3.6 pct of Hg extraction was attained in 4 h with 0.63 lb sodium sulfide.

