Table of Contents
The process of treating granular activated carbon for return to service in the recovery of gold in Carbon in Pulp and Carbon in Leach circuits has been investigated. Carbons from four U.S.A. mines, one Canadian mine and one South African mine were characterized before and after regeneration. The use of thermal gravimetric analyses demonstrated the presence of inorganic compounds on the surface of the carbon. Thermal regeneration was investigated using factorial analysis to determine the effect or interaction of furnace temperature, retention time and the use of supplemental steam, the operation of acid leaching prior to or after gold elution was investigated. The effect of water quenching regenerated carbon was also studied. From the data obtained in this investigation, guidelines are given for effective regeneration operation.
Experimental
The carbon samples from the South African CIL pilot plant were stripped by contacting them with 3% HCl at ambient conditions for one hour. This was followed by a neutralization with 1% Na2CO3 at ambient conditions and two bed volumes (B.V.) of distilled water (D.I.) at 120 °C. Gold elution of the carbon samples was performed using a pressurized system by pumping a 2% NaCN/1% Na2CO3 solution through the carbon at 120°C until an alkaline pH (≈ 11) was detected. The carbon was then allowed to soak for one hour maintaining a temperature of 120°C. After one hour, seven bed volumes of distilled water were pumped through the carbon bed also at 120°C.
All thermal regenerations were conducted using a two-inch externally heated rotary tube furnace. In order to simulate an expected plant condition of 50 percent moisture in the carbon prior to regeneration, 100g of oven dried material was placed in a 250 ml jar and 50g of D.I. water was added. The jar was then tightly capped and the carbon allowed to equilibrate.
Lab quenching consisted of pouring the hot regenerated product immediately after furnace discharge into 1,000 ml of D.I. water contained in a 3L metal beaker. A portion of the quenched carbon was dried at 120°C in a nitrogen gas environment to eliminate any possible chemisorption of oxygen. Another portion was dried at 120°C in a lab oven with no attempt to control the environment.
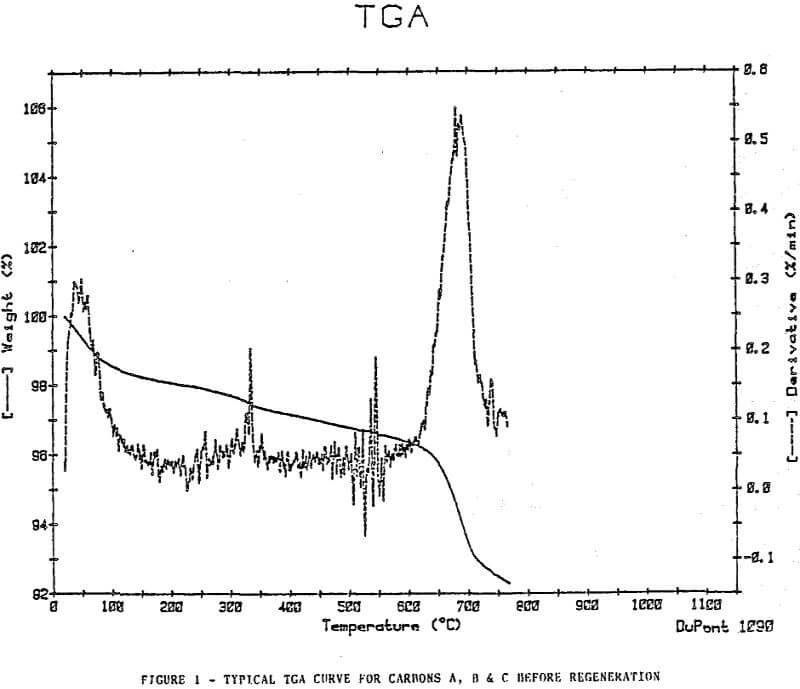
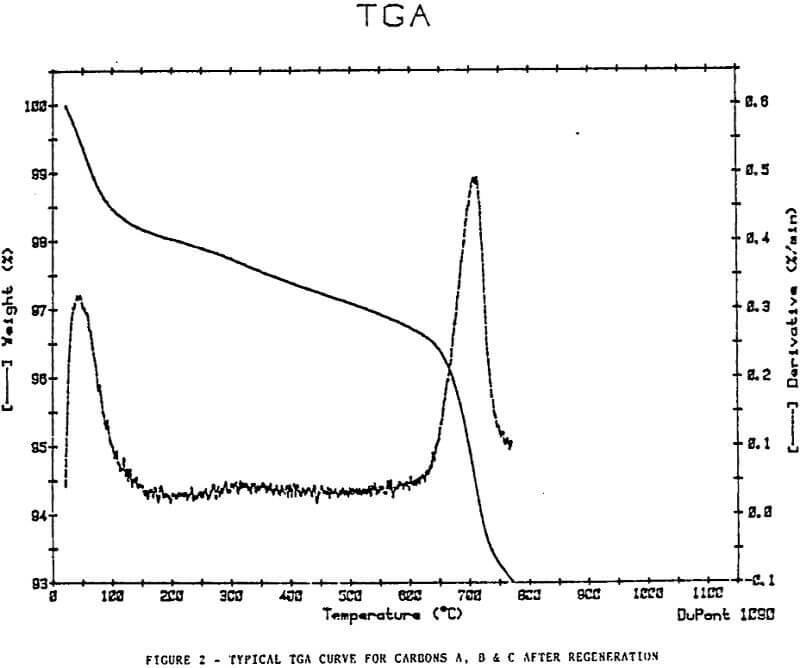
Thermal Gravimetric Analyses
The plots show removal of moisture at approximately 100°C, a slow decline in weight at a steady rate to approximately 500-600°c, then an increase in weight loss rate at a temperature >600°C.
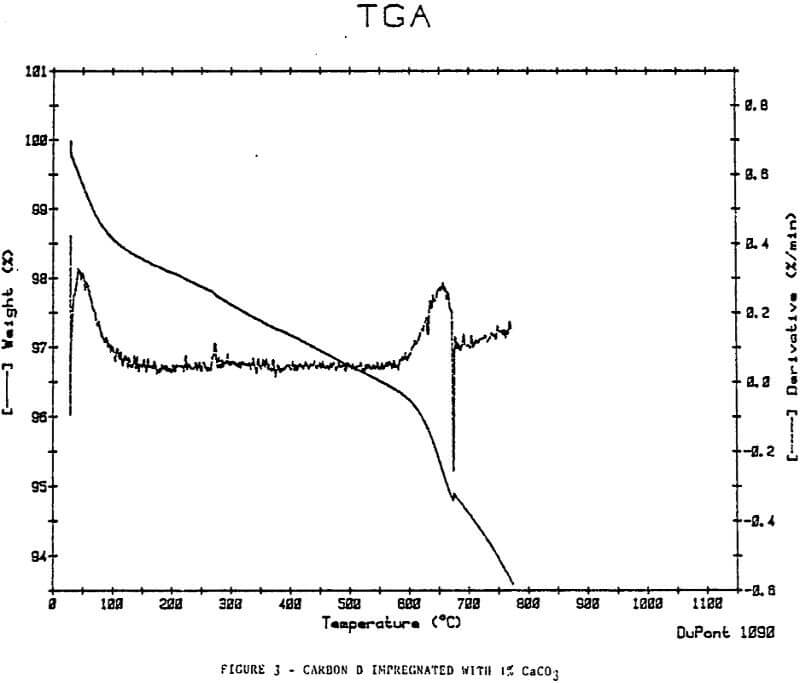
In an attempt to simulate inorganic loading on carbon, the calcium ion, which is abundant in CIP and CIL processes, was impregnated on a sample of regenerated Carbon D using Ca(OH)2 (1% Wt Ca). The carbon was then exposed to CO2. The regenerated Carbon D did not show the weight loss increase at 600-700°C.
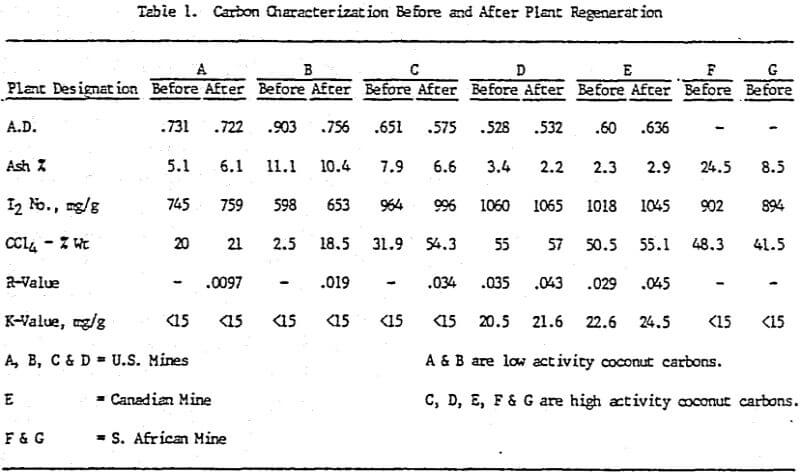
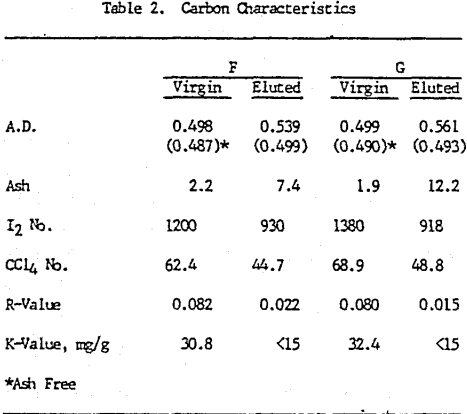
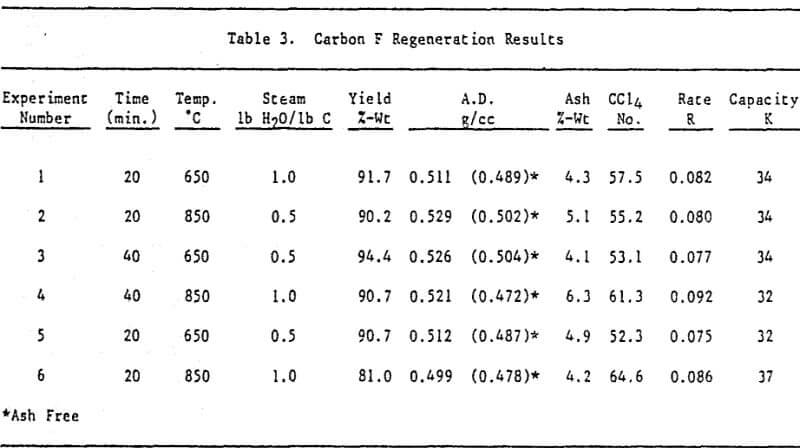
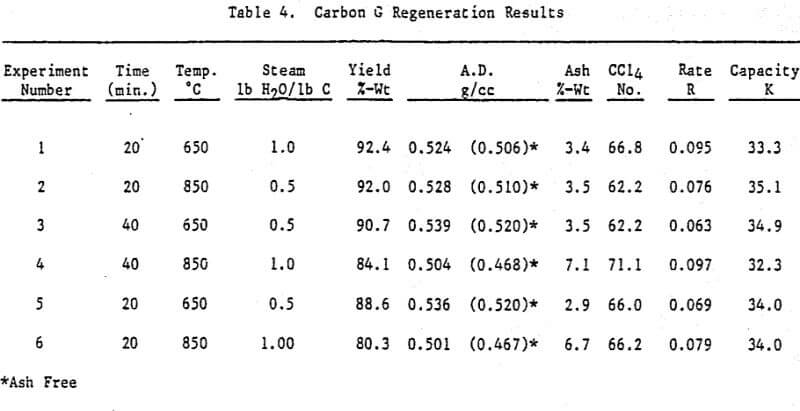
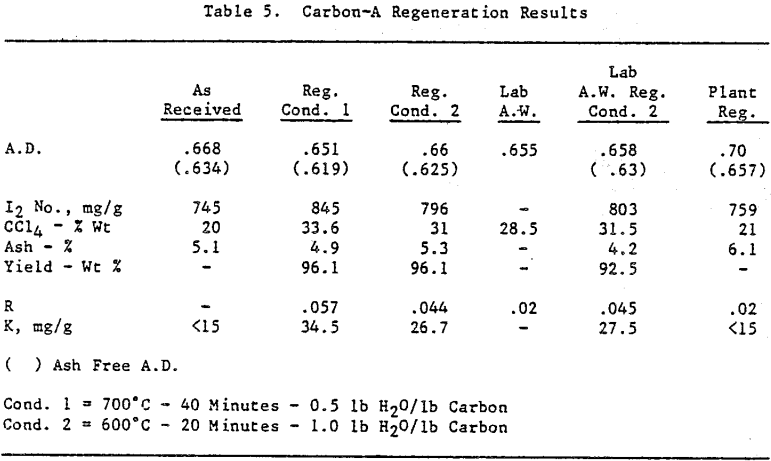
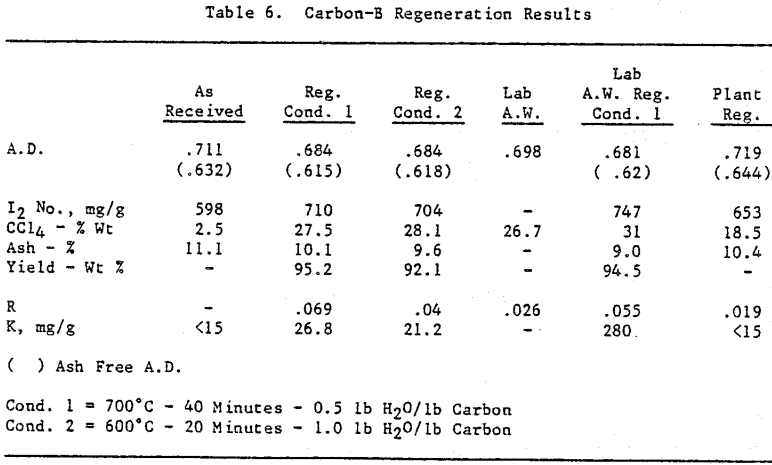
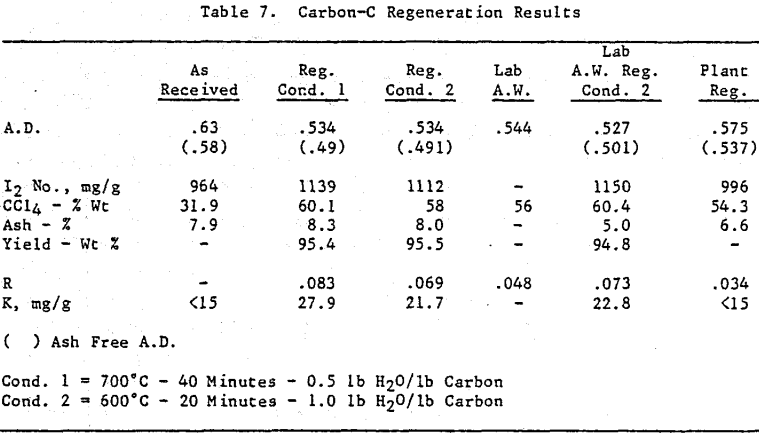
Initially, a factorial experiment was designed to determine the effect of furnace retention time, regeneration temperature and steam addition. Prior to regeneration, the loaded carbon samples were laboratory stripped and eluted. After the HCl strip and elution with a Na2CO3/NaCN solution using the previously described procedure, ash was reduced slightly with minor increases in CCl4 and Iodine Number. Adsorption rate (R) was significantly lower than virgin carbon.
Based on the results of the factorial experiments, two conditions were selected to perform lab regenerations on samples A-E.
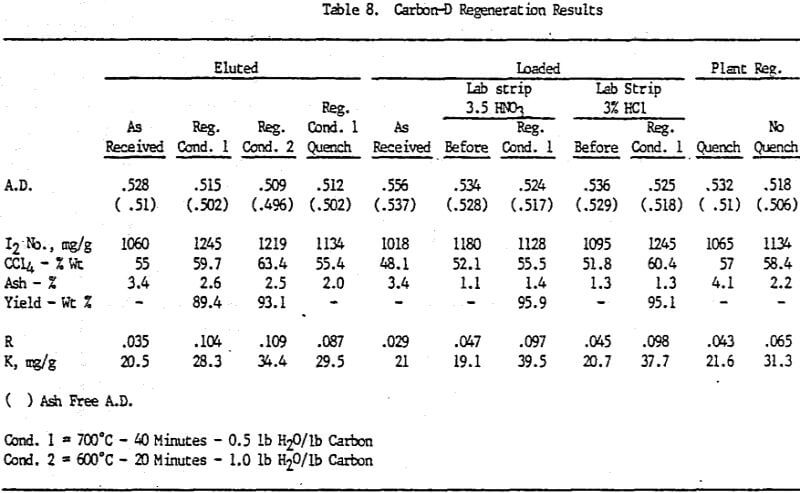
Analyses of samples from mine D show that the carbon operation at this mine is the best of all the mines investigated.
Carbon samples of loaded, eluted and plant regenerated with and without water quenching were received from plant D. This plant includes an acid wash step prior to gold elution. Laboratory elutions were conducted to evaluate the effects, if any, of acid washing with HCl versus HNO3.